High phosphate induces skeletal muscle atrophy and suppresses myogenic differentiation by increasing oxidative stress and activating Nrf2 signaling
- PMID: 33136552
- PMCID: PMC7695395
- DOI: 10.18632/aging.103896
High phosphate induces skeletal muscle atrophy and suppresses myogenic differentiation by increasing oxidative stress and activating Nrf2 signaling
Abstract
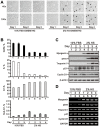
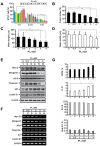
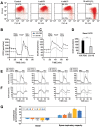
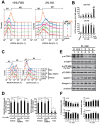
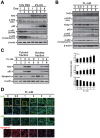
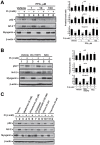
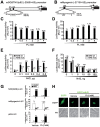
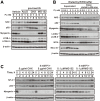
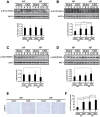
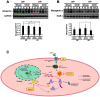
Skeletal muscle wasting represents both a common phenotype of aging and a feature of pathological conditions such as chronic kidney disease (CKD). Although both clinical data and genetic experiments in mice suggest that hyperphosphatemia accelerates muscle wasting, the underlying mechanism remains unclear. Here, we showed that inorganic phosphate (Pi) dose-dependently decreases myotube size, fusion index, and myogenin expression in mouse C2C12 skeletal muscle cells. These changes were accompanied by increases in reactive oxygen species (ROS) production and Nrf2 and p62 expression, and reductions in mitochondrial membrane potential (MMP) and Keap1 expression. Inhibition of Pi entry, cytosolic ROS production, or Nrf2 activation reversed the effects of high Pi on Nrf2, p62, and myogenin expression. Overexpression of Nrf2 respectively increased and decreased the promoter activity of p62-Luc and myogenin-Luc reporters. Analysis of nuclear extracts from gastrocnemius muscles from mice fed a high-Pi (2% Pi) diet showed increased Nrf2 phosphorylation in sham-operated and 5/6 nephrectomized (CKD) mice, and both increased p62 phosphorylation and decreased myogenin expression in CKD mice. These data suggest that high Pi suppresses myogenic differentiation in vitro and promotes muscle atrophy in vivo through oxidative stress-mediated protein degradation and both canonical (ROS-mediated) and non-canonical (p62-mediated) activation of Nrf2 signaling.
Keywords: Nrf2; hyperphosphatemia; muscle wasting; myogenic differentiation; oxidative stress.
Conflict of interest statement
Figures
References
-
- McIntyre CW, Selby NM, Sigrist M, Pearce LE, Mercer TH, Naish PF. Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol Dial Transplant. 2006; 21:2210–16. 10.1093/ndt/gfl064 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

