Effects of Head Movements on Sound-Source Localization in Single-Sided Deaf Patients With Their Cochlear Implant On Versus Off
- PMID: 33136640
- PMCID: PMC7772279
- DOI: 10.1097/AUD.0000000000000882
Effects of Head Movements on Sound-Source Localization in Single-Sided Deaf Patients With Their Cochlear Implant On Versus Off
Abstract
Objectives: We investigated the ability of single-sided deaf listeners implanted with a cochlear implant (SSD-CI) to (1) determine the front-back and left-right location of sound sources presented from loudspeakers surrounding the listener and (2) use small head rotations to further improve their localization performance. The resulting behavioral data were used for further analyses investigating the value of so-called "monaural" spectral shape cues for front-back sound source localization.
Design: Eight SSD-CI patients were tested with their cochlear implant (CI) on and off. Eight normal-hearing (NH) listeners, with one ear plugged during the experiment, and another group of eight NH listeners, with neither ear plugged, were also tested. Gaussian noises of 3-sec duration were band-pass filtered to 2-8 kHz and presented from 1 of 6 loudspeakers surrounding the listener, spaced 60° apart. Perceived sound source localization was tested under conditions where the patients faced forward with the head stationary, and under conditions where they rotated their heads between (Equation is included in full-text article.).
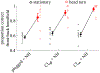
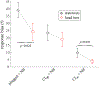
Results: (1) Under stationary listener conditions, unilaterally-plugged NH listeners and SSD-CI listeners (with their CIs both on and off) were nearly at chance in determining the front-back location of high-frequency sound sources. (2) Allowing rotational head movements improved performance in both the front-back and left-right dimensions for all listeners. (3) For SSD-CI patients with their CI turned off, head rotations substantially reduced front-back reversals, and the combination of turning on the CI with head rotations led to near-perfect resolution of front-back sound source location. (4) Turning on the CI also improved left-right localization performance. (5) As expected, NH listeners with both ears unplugged localized to the correct front-back and left-right hemifields both with and without head movements.
Conclusions: Although SSD-CI listeners demonstrate a relatively poor ability to distinguish the front-back location of sound sources when their head is stationary, their performance is substantially improved with head movements. Most of this improvement occurs when the CI is off, suggesting that the NH ear does most of the "work" in this regard, though some additional gain is introduced with turning the CI on. During head turns, these listeners appear to primarily rely on comparing changes in head position to changes in monaural level cues produced by the direction-dependent attenuation of high-frequency sounds that result from acoustic head shadowing. In this way, SSD-CI listeners overcome limitations to the reliability of monaural spectral and level cues under stationary conditions. SSD-CI listeners may have learned, through chronic monaural experience before CI implantation, or with the relatively impoverished spatial cues provided by their CI-implanted ear, to exploit the monaural level cue. Unilaterally-plugged NH listeners were also able to use this cue during the experiment to realize approximately the same magnitude of benefit from head turns just minutes after plugging, though their performance was less accurate than that of the SSD-CI listeners, both with and without their CI turned on.
Figures


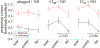


References
-
- Agterberg MJ, Hol MK, Van Wanrooij M, Van Opstal AJ, and Snik AF (2014). “Single-sided deafness and directional hearing: contribution of spectral cues and high-frequency hearing loss in the hearing ear,” Front Neurosci 8, 188, https://www.ncbi.nlm.nih.gov/pubmed/25071433, doi: 10.3389/fnins.2014.00188. - DOI - PMC - PubMed
-
- Bauer RW, Matuza JL, and Blackmer RF (1966). “Noise localization after unilateral attenuation,” J Acoust Soc Am 40(2), 441–444.
-
- Bronkhorst AW (2015). “The cocktail-party problem revisited: early processing and selection of multi-talker speech,” Atten Percept Psychophys http://link.springer.com/10.3758/s13414-015-0882-9, doi: 10.3758/s13414-015-0882-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

