Docosahexaenoic acid inhibits the proliferation of Kras/TP53 double mutant pancreatic ductal adenocarcinoma cells through modulation of glutathione level and suppression of nucleotide synthesis
- PMID: 33137095
- PMCID: PMC7605869
- DOI: 10.1371/journal.pone.0241186
Docosahexaenoic acid inhibits the proliferation of Kras/TP53 double mutant pancreatic ductal adenocarcinoma cells through modulation of glutathione level and suppression of nucleotide synthesis
Abstract
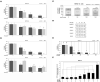
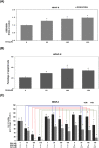
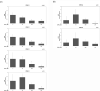
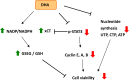
The treatment of cancer cells obtained by blocking cellular metabolism has received a lot of attention recently. Previous studies have demonstrated that Kras mutation-mediated abnormal glucose metabolism would lead to an aberrant cell proliferation in human pancreatic ductal adenocarcinoma (PDAC) cells. Previous literature has suggested that consumption of fish oil is associated with lower risk of pancreatic cancer. In this study, we investigated the anti-cancer effects of docosahexaenoic acid (DHA) in human PDAC cells in vitro and in vivo. Omega-3 polyunsaturated fatty acids (PUFAs) such as DHA and eicosapentaenoic acid (EPA) significantly inhibited the proliferation of human PDAC cells. The actions of DHA were evaluated through an induction of cell cycle arrest at G1 phase and noticed a decreased expression of cyclin A, cyclin E and cyclin B proteins in HPAF-II cells. Moreover, it was found that co-treatment of DHA and gemcitabine (GEM) effectively induced oxidative stress and cell death in HPAF-II cells. Interestingly, DHA leads to an increased oxidative glutathione /reduced glutathione (GSSG/GSH) ratio and induced cell apoptosis in HPAF-II cells. The findings in the study showed that supplementation of GSH or N-Acetyl Cysteine (NAC) could reverse DHA-mediated cell death in HPAF-II cells. Additionally, DHA significantly increased cellular level of cysteine, cellular NADP/NADPH ratio and the expression of cystathionase (CTH) and SLCA11/xCT antiporter proteins in HPAF-II cells. The action of DHA was, in part, associated with the inactivation of STAT3 cascade in HPAF-II cells. Treatment with xCT inhibitors, such as erastin or sulfasalazine (SSZ), inhibited the cell survival ability in DHA-treated HPAF-II cells. DHA also inhibited nucleotide synthesis in HPAF-II cells. It was demonstrated in a mouse-xenograft model that consumption of fish oil significantly inhibited the growth of pancreatic adenocarcinoma and decreased cellular nucleotide level in tumor tissues. Furthermore, fish oil consumption induced an increment of GSSG/GSH ratio, an upregulation of xCT and CTH proteins in tumor tissues. In conclusion, DHA significantly inhibited survival of PDAC cells both in vitro and in vivo through its recently identified novel mode of action, including an increment in the ratio of GSSG/GSH and NADP/NADPH respectively, and promoting reduction in the levels of nucleotide synthesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Docosahexaenoic Acid Inhibits Cell Proliferation through a Suppression of c-Myc Protein in Pancreatic Ductal Adenocarcinoma Cells.Antioxidants (Basel). 2021 Oct 28;10(11):1721. doi: 10.3390/antiox10111721. Antioxidants (Basel). 2021. PMID: 34829591 Free PMC article.
-
Eicosapentaenoic Acid Inhibits KRAS Mutant Pancreatic Cancer Cell Growth by Suppressing Hepassocin Expression and STAT3 Phosphorylation.Biomolecules. 2021 Mar 2;11(3):370. doi: 10.3390/biom11030370. Biomolecules. 2021. PMID: 33801246 Free PMC article.
-
Docosahexaenoic acid induces apoptosis in the human PaCa-44 pancreatic cancer cell line by active reduced glutathione extrusion and lipid peroxidation.Nutr Cancer. 2005;52(2):225-33. doi: 10.1207/s15327914nc5202_12. Nutr Cancer. 2005. PMID: 16201853
-
Pancreatic Cancer Research beyond DNA Mutations.Biomolecules. 2022 Oct 17;12(10):1503. doi: 10.3390/biom12101503. Biomolecules. 2022. PMID: 36291712 Free PMC article. Review.
-
Cell death in pancreatic cancer: from pathogenesis to therapy.Nat Rev Gastroenterol Hepatol. 2021 Nov;18(11):804-823. doi: 10.1038/s41575-021-00486-6. Epub 2021 Jul 30. Nat Rev Gastroenterol Hepatol. 2021. PMID: 34331036 Review.
Cited by
-
Genome, Metabolism, or Immunity: Which Is the Primary Decider of Pancreatic Cancer Fate through Non-Apoptotic Cell Death?Biomedicines. 2023 Oct 14;11(10):2792. doi: 10.3390/biomedicines11102792. Biomedicines. 2023. PMID: 37893166 Free PMC article. Review.
-
Sulfasalazine Sensitizes Polyhematoporphyrin-Mediated Photodynamic Therapy in Cholangiocarcinoma by Targeting xCT.Front Pharmacol. 2021 Aug 13;12:723488. doi: 10.3389/fphar.2021.723488. eCollection 2021. Front Pharmacol. 2021. PMID: 34483935 Free PMC article.
-
Docosahexaenoic Acid Inhibits Cell Proliferation through a Suppression of c-Myc Protein in Pancreatic Ductal Adenocarcinoma Cells.Antioxidants (Basel). 2021 Oct 28;10(11):1721. doi: 10.3390/antiox10111721. Antioxidants (Basel). 2021. PMID: 34829591 Free PMC article.
-
d-α-tocopheryl polyethylene glycol 1000 succinate surface scaffold polysarcosine based polymeric nanoparticles of enzalutamide for the treatment of colorectal cancer: In vitro, in vivo characterizations.Heliyon. 2024 Feb 2;10(3):e25172. doi: 10.1016/j.heliyon.2024.e25172. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38333874 Free PMC article.
References
-
- Modolell I, Guarner L, Malagelada JR. Vagaries of clinical presentation of pancreatic and biliary tract cancer. Annals of oncology: official journal of the European Society for Medical Oncology. 1999;10 Suppl 4:82–4. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

