Membrane progesterone receptor induces meiosis in Xenopus oocytes through endocytosis into signaling endosomes and interaction with APPL1 and Akt2
- PMID: 33137110
- PMCID: PMC7660923
- DOI: 10.1371/journal.pbio.3000901
Membrane progesterone receptor induces meiosis in Xenopus oocytes through endocytosis into signaling endosomes and interaction with APPL1 and Akt2
Erratum in
-
Correction: Membrane progesterone receptor induces meiosis in Xenopus oocytes through endocytosis into signaling endosomes and interaction with APPL1 and Akt2.PLoS Biol. 2021 Feb 10;19(2):e3001117. doi: 10.1371/journal.pbio.3001117. eCollection 2021 Feb. PLoS Biol. 2021. PMID: 33566812 Free PMC article.
Abstract
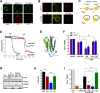
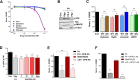
The steroid hormone progesterone (P4) mediates many physiological processes through either nuclear receptors that modulate gene expression or membrane P4 receptors (mPRs) that mediate nongenomic signaling. mPR signaling remains poorly understood. Here we show that the topology of mPRβ is similar to adiponectin receptors and opposite to that of G-protein-coupled receptors (GPCRs). Using Xenopus oocyte meiosis as a well-established physiological readout of nongenomic P4 signaling, we demonstrate that mPRβ signaling requires the adaptor protein APPL1 and the kinase Akt2. We further show that P4 induces clathrin-dependent endocytosis of mPRβ into signaling endosome, where mPR interacts transiently with APPL1 and Akt2 to induce meiosis. Our findings outline the early steps involved in mPR signaling and expand the spectrum of mPR signaling through the multitude of pathways involving APPL1.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: KM is a co-founder of Valdia Health.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

