TOR Complex 2- independent mutations in the regulatory PIF pocket of Gad8AKT1/SGK1 define separate branches of the stress response mechanisms in fission yeast
- PMID: 33137119
- PMCID: PMC7660925
- DOI: 10.1371/journal.pgen.1009196
TOR Complex 2- independent mutations in the regulatory PIF pocket of Gad8AKT1/SGK1 define separate branches of the stress response mechanisms in fission yeast
Abstract
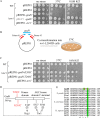
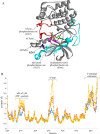
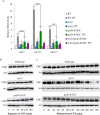
The Target of rapamycin (TOR) protein kinase forms part of TOR complex 1 (TORC1) and TOR complex 2 (TORC2), two multi-subunit protein complexes that regulate growth, proliferation, survival and developmental processes by phosphorylation and activation of AGC-family kinases. In the fission yeast, Schizosaccharomyces pombe, TORC2 and its target, the AGC kinase Gad8 (an orthologue of human AKT or SGK1) are required for viability under stress conditions and for developmental processes in response to starvation cues. In this study, we describe the isolation of gad8 mutant alleles that bypass the requirement for TORC2 and reveal a separation of function of TORC2 and Gad8 under stress conditions. In particular, osmotic and nutritional stress responses appear to form a separate branch from genotoxic stress responses downstream of TORC2-Gad8. Interestingly, TORC2-independent mutations map into the regulatory PIF pocket of Gad8, a highly conserved motif in AGC kinases that regulates substrate binding in PDK1 (phosphoinositide dependent kinase-1) and kinase activity in several AGC kinases. Gad8 activation is thought to require a two-step mechanism, in which phosphorylation by TORC2 allows further phosphorylation and activation by Ksg1 (an orthologue of PDK1). We focus on the Gad8-K263C mutation and demonstrate that it renders the Gad8 kinase activity independent of TORC2 in vitro and independent of the phosphorylation sites of TORC2 in vivo. Molecular dynamics simulations of Gad8-K263C revealed abnormal high flexibility at T387, the phosphorylation site for Ksg1, suggesting a mechanism for the TORC2-independent Gad8 activity. Significantly, the K263 residue is highly conserved in the family of AGC-kinases, which may suggest a general way of keeping their activity in check when acting downstream of TOR complexes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

