Performance of nucleocapsid and spike-based SARS-CoV-2 serologic assays
- PMID: 33137138
- PMCID: PMC7605638
- DOI: 10.1371/journal.pone.0237828
Performance of nucleocapsid and spike-based SARS-CoV-2 serologic assays
Abstract
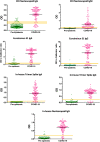
There is an urgent need for an accurate antibody test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We have developed 3 ELISA methods, trimer spike IgA, trimer spike IgG, and nucleocapsid IgG, for detecting anti-SARS-CoV-2 antibodies. We evaluated their performance along with four commercial ELISAs, EDI™ Novel Coronavirus COVID-19 ELISA IgG and IgM, Euroimmun Anti-SARS-CoV-2 ELISA IgG and IgA, and one lateral flow assay, DPP® COVID-19 IgM/IgG System (Chembio). Both sensitivity and specificity were evaluated and the probable causes of false-positive reactions were determined. The assays were evaluated using 300 pre-epidemic samples and 100 PCR-confirmed COVID-19 samples. The sensitivities and specificities of the assays were as follows: 90%/100% (in-house trimer spike IgA), 90%/99.3% (in-house trimer spike IgG), 89%/98.3% (in-house nucleocapsid IgG), 73.7%/100% (EDI nucleocapsid IgM), 84.5%/95.1% (EDI nucleocapsid IgG), 95%/93.7% (Euroimmun S1 IgA), 82.8%/99.7% (Euroimmun S1 IgG), 82.0%/91.7% (Chembio nucleocapsid IgM), 92%/93.3% (Chembio nucleocapsid IgG). The presumed causes of false positive results from pre-epidemic samples in commercial and in-house assays were mixed. In some cases, assays lacked reproducibility. In other cases, reactivity was abrogated by competitive inhibition (spiking the sample with the same antigen that was used for coating ELISAs prior to performing the assay), suggesting positive reaction could be attributed to the presence of antibodies against these antigens. In other cases, reactivity was consistently detected but not abrogated by the spiking, suggesting positive reaction was not attributed to the presence of antibodies against these antigens. Overall, there was wide variability in assay performance using our samples, with in-house tests exhibiting the highest combined sensitivity and specificity. The causes of "false positivity" in pre-epidemic samples may be due to plasma antibodies apparently reacting with the corresponding antigen, or spurious reactivity may be directed against non-specific components in the assay system. Identification of these targets will be essential to improving assay performance.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Update of
-
Specificity and Performance of Nucleocapsid and Spike-based SARS-CoV-2 Serologic Assays.medRxiv [Preprint]. 2020 Aug 7:2020.08.05.20168476. doi: 10.1101/2020.08.05.20168476. medRxiv. 2020. Update in: PLoS One. 2020 Nov 2;15(11):e0237828. doi: 10.1371/journal.pone.0237828. PMID: 32793933 Free PMC article. Updated. Preprint.
Similar articles
-
Specificity and Performance of Nucleocapsid and Spike-based SARS-CoV-2 Serologic Assays.medRxiv [Preprint]. 2020 Aug 7:2020.08.05.20168476. doi: 10.1101/2020.08.05.20168476. medRxiv. 2020. Update in: PLoS One. 2020 Nov 2;15(11):e0237828. doi: 10.1371/journal.pone.0237828. PMID: 32793933 Free PMC article. Updated. Preprint.
-
Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by four automated immunoassays and three ELISAs.Clin Microbiol Infect. 2020 Nov;26(11):1557.e1-1557.e7. doi: 10.1016/j.cmi.2020.07.038. Epub 2020 Jul 31. Clin Microbiol Infect. 2020. PMID: 32745595 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2020 Jun 25;6(6):CD013652. doi: 10.1002/14651858.CD013652. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2022 Nov 17;11:CD013652. doi: 10.1002/14651858.CD013652.pub2. PMID: 32584464 Free PMC article. Updated.
-
SARS-CoV-2 S1 and N-based serological assays reveal rapid seroconversion and induction of specific antibody response in COVID-19 patients.Sci Rep. 2020 Oct 6;10(1):16561. doi: 10.1038/s41598-020-73491-5. Sci Rep. 2020. PMID: 33024213 Free PMC article.
-
Reflections on COVID-19: A Literature Review of SARS-CoV-2 Testing.Vaccines (Basel). 2024 Dec 26;13(1):9. doi: 10.3390/vaccines13010009. Vaccines (Basel). 2024. PMID: 39852788 Free PMC article. Review.
Cited by
-
COVID-19 Pandemic: Review of Contemporary and Forthcoming Detection Tools.Infect Drug Resist. 2021 Mar 17;14:1049-1082. doi: 10.2147/IDR.S289629. eCollection 2021. Infect Drug Resist. 2021. PMID: 33762831 Free PMC article. Review.
-
Development of an Affordable ELISA Targeting the SARS-CoV-2 Nucleocapsid and Its Application to Samples from the Ongoing COVID-19 Epidemic in Ghana.Mol Diagn Ther. 2023 Sep;27(5):583-592. doi: 10.1007/s40291-023-00655-0. Epub 2023 Jul 18. Mol Diagn Ther. 2023. PMID: 37462793 Free PMC article.
-
A novel highly specific biotinylated MAC-ELISA for detection of anti-SARS-CoV-2 nucleocapsid antigen IgM antibodies during the acute phase of COVID-19.Braz J Microbiol. 2023 Dec;54(4):2893-2901. doi: 10.1007/s42770-023-01160-6. Epub 2023 Nov 6. Braz J Microbiol. 2023. PMID: 37930615 Free PMC article.
-
Diagnostic performance of two serological assays for the detection of SARS-CoV-2 specific antibodies: surveillance after vaccination.Diagn Microbiol Infect Dis. 2022 Apr;102(4):115650. doi: 10.1016/j.diagmicrobio.2022.115650. Epub 2022 Jan 26. Diagn Microbiol Infect Dis. 2022. PMID: 35218991 Free PMC article.
-
Prevalence of anti-severe acute respiratory syndrome coronavirus 2 antibodies in cats in Germany and other European countries in the early phase of the coronavirus disease-19 pandemic.Zoonoses Public Health. 2022 Aug;69(5):439-450. doi: 10.1111/zph.12932. Epub 2022 Mar 2. Zoonoses Public Health. 2022. PMID: 35238485 Free PMC article.
References
-
- Testing, Screening, and Outbreak Response for Institutions of Higher Education (IHEs): Centers for Disease Control and Prevention (CDC); [updated Sept. 30, 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/community/colleges-universitie....
-
- Interim Guidelines for COVID-19 Antibody Testing CDC: Center for Disease Control and Prevention; Updated Aug. 1, 2020 [10.09.2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-g....
-
- Nicol T, Lefeuvre C, Serri O, Pivert A, Joubaud F, Dubee V, et al. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: Two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech). J Clin Virol. 2020;129:104511 Epub 2020/06/28. 10.1016/j.jcv.2020.104511 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

