Cyanine Dyes Containing Quinoline Moieties: History, Synthesis, Optical Properties, and Applications
- PMID: 33137212
- PMCID: PMC9832344
- DOI: 10.1002/chem.202003697
Cyanine Dyes Containing Quinoline Moieties: History, Synthesis, Optical Properties, and Applications
Erratum in
-
Corrigendum: Cyanine Dyes Containing Quinoline Moieties: History, Synthesis, Optical Properties, and Applications.Chemistry. 2022 Apr 22;28(23):e202104530. doi: 10.1002/chem.202104530. Epub 2022 Apr 5. Chemistry. 2022. PMID: 35384095 No abstract available.
Abstract
Cyanine dyes carrying quinoline moieties are an important class of organic molecules that are of great interest for applications in many fields like medicine, pharmacology, and engineering. Despite their exceptional properties, such as stability, high molar extinction coefficients, and high pH-sensitivity, this class of dyes has been less analyzed and reviewed in the last few decades. Therefore, this review article focuses on discussing the history of quinoline compounds, various synthetic routes to prepare quinolinium salts and symmetrical and asymmetrical mono-, di-, tri-, penta- and heptamethine cyanine dyes, containing quinoline moieties, together with their optical properties and applications as photosensitizers in photodynamic therapy, probes in biomolecules for labeling of nucleic acids, as well as imaging agents.
Keywords: NIR; cyanine dyes; lepidine; quinaldine; quinoline.
© 2020 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures


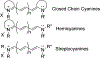

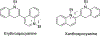
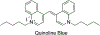


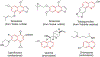

























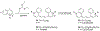
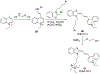


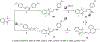






References
-
- Ullah S, Ahmad I, Alias Y, Yusoff I, Ashraf MA, J. Chem 2013, 136908.
-
- Ma X, Laramie M, Henary M, Bioorg. Med. Chem. Lett 2018, 28, 509–514. - PubMed
-
- Henary M, Paranjpe S, Owens EA, Heterocycl. Commun 2013, 19, 1–11.
-
- Henary M, Levitz A, Dyes Pigm. 2013, 99, 1107–1116.
-
- Visible/Infrared Spectroscopy (VIRS) as a Research Tool in Economic Geology: Background and Pilot Studies from Newfoundland and Labrador (Eds.: Kerr A, Rafuse H, Sparkes GW, Hinchey J, Sandeman H), Current Research Newfoundland and Labrador Department of Natural Resources Geological Survey, Report 11–1, 2011, pp. 145–166.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

