Advanced spray dried proliposomes of amphotericin B lung surfactant-mimic phospholipid microparticles/nanoparticles as dry powder inhalers for targeted pulmonary drug delivery
- PMID: 33137515
- PMCID: PMC8923534
- DOI: 10.1016/j.pupt.2020.101975
Advanced spray dried proliposomes of amphotericin B lung surfactant-mimic phospholipid microparticles/nanoparticles as dry powder inhalers for targeted pulmonary drug delivery
Abstract
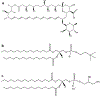
The purpose of this study was to design, develop and characterize inhalable proliposomal microparticles/nanoparticles of Amphotericin B (AmB) with synthetic phospholipids, dipalmitoylphosphatidylcholine (DPPC) and dipalmitoylphosphatidylglycerol (DPPG) which are lung surfactant-mimic phospholipids. Organic solutions of AmB and phospholipids, were co-spray dried using an advanced closed-mode system and a high performance cyclone. Scanning electron microscopy (SEM) was employed to visualize the surface structure, morphology, and particles size. The residual water content of the proliposomes was quantified by Karl Fisher coulometric titration (KFT). Degree of crystallinity/non-crystallinity was measured by X-ray powder diffraction (XRPD). Phase behavior was measured by differential scanning calorimetry. The chemical composition by molecular fingerprinting was established using attenuated total reflectance (ATR)-Fourier-transform infrared (FTIR) spectroscopy. The amount of AmB loaded into the proliposomes was quantified using UV-VIS spectroscopy. The in vitro aerosol dispersion performance was conducted using the Next Generation Impactor (NGI) and the human dry powder inhaler (DPI) (Handihaler®) that is FDA-approved. Different human lung cell lines were employed to demonstrate in vitro safety as a function of dose and formulation. Smooth, spherical microparticles/nanoparticles were formed at medium and high spray drying pump rates and had low residual water content. A characteristic peak in the XRPD diffraction pattern as well as an endotherm in DSC confirmed the presence of the lipid bilayer structure characteristic in the DPPC/DPPG proliposomal systems. Superior in vitro aerosol performance was achieved with engineered microparticles/nanoparticles demonstrating suitability for targeted pulmonary drug delivery as inhalable dry powders. The in vitro cellular studies demonstrated that the formulated proliposomes are safe. These AmB proliposomes can be a better option for targeted treatment of severe pulmonary fungal infections.
Keywords: Co-spray drying particle engineering; Human inhaler device; In vitro aerosol dispersion; In vitro human pulmonary cell viability; Lung surfactant phospholipids; Pulmonary drug delivery.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures





Similar articles
-
Design, physicochemical characterization, and optimization of organic solution advanced spray-dried inhalable dipalmitoylphosphatidylcholine (DPPC) and dipalmitoylphosphatidylethanolamine poly(ethylene glycol) (DPPE-PEG) microparticles and nanoparticles for targeted respiratory nanomedicine delivery as dry powder inhalation aerosols.Int J Nanomedicine. 2013;8:275-93. doi: 10.2147/IJN.S30724. Epub 2013 Jan 15. Int J Nanomedicine. 2013. PMID: 23355776 Free PMC article.
-
Characterization and aerosol dispersion performance of advanced spray-dried chemotherapeutic PEGylated phospholipid particles for dry powder inhalation delivery in lung cancer.Eur J Pharm Sci. 2013 Jul 16;49(4):699-711. doi: 10.1016/j.ejps.2013.05.012. Epub 2013 May 23. Eur J Pharm Sci. 2013. PMID: 23707466 Free PMC article.
-
High-performing dry powder inhalers of paclitaxel DPPC/DPPG lung surfactant-mimic multifunctional particles in lung cancer: physicochemical characterization, in vitro aerosol dispersion, and cellular studies.AAPS PharmSciTech. 2014 Dec;15(6):1574-87. doi: 10.1208/s12249-014-0182-z. Epub 2014 Aug 20. AAPS PharmSciTech. 2014. PMID: 25139763 Free PMC article.
-
A Review on Micro and Nanoengineering in Powder-Based Pulmonary Drug Delivery.Int J Pharm. 2024 Jun 25;659:124248. doi: 10.1016/j.ijpharm.2024.124248. Epub 2024 May 21. Int J Pharm. 2024. PMID: 38782150 Review.
-
Solid state of inhalable high dose powders.Adv Drug Deliv Rev. 2022 Oct;189:114468. doi: 10.1016/j.addr.2022.114468. Epub 2022 Jul 30. Adv Drug Deliv Rev. 2022. PMID: 35917868 Review.
Cited by
-
Inhaled Antifungal Agents for Treatment and Prophylaxis of Bronchopulmonary Invasive Mold Infections.Pharmaceutics. 2022 Mar 14;14(3):641. doi: 10.3390/pharmaceutics14030641. Pharmaceutics. 2022. PMID: 35336015 Free PMC article. Review.
-
Comprehensive Physicochemical Characterization, In Vitro Membrane Permeation, and In Vitro Human Skin Cell Culture of a Novel TOPK Inhibitor, HI-TOPK-032.Int J Mol Sci. 2023 Oct 24;24(21):15515. doi: 10.3390/ijms242115515. Int J Mol Sci. 2023. PMID: 37958502 Free PMC article.
-
Inhalable microparticles as drug delivery systems to the lungs in a dry powder formulations.Regen Biomater. 2022 Dec 8;10:rbac099. doi: 10.1093/rb/rbac099. eCollection 2023. Regen Biomater. 2022. PMID: 36683752 Free PMC article. Review.
-
Design and comprehensive characterization of dry powder inhalation aerosols of simvastatin DPPC/DPPG lung surfactant-mimic nanoparticles/microparticles for pulmonary nanomedicine.RSC Adv. 2024 Sep 16;14(40):29413-29427. doi: 10.1039/d4ra04947k. eCollection 2024 Sep 12. RSC Adv. 2024. PMID: 39285876 Free PMC article.
-
A Phase I/IIa Prospective, Randomized, Open-Label Study on the Safety and Efficacy of Nebulized Liposomal Amphotericin for Invasive Pulmonary Aspergillosis.J Fungi (Basel). 2024 Mar 1;10(3):191. doi: 10.3390/jof10030191. J Fungi (Basel). 2024. PMID: 38535200 Free PMC article.
References
-
- Herbrecht R; Denning DW; Patterson TF; Bennett JE; Greene RE; Oestmann J-W; Kern WV; Marr KA; Ribaud P; Lortholary O Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. New England Journal of Medicine 2002, 347, (6), 408–415. - PubMed
-
- Walsh TJ; Anaissie EJ; Denning DW; Herbrecht R; Kontoyiannis DP; Marr KA; Morrison VA; Segal BH; Steinbach WJ; Stevens DA Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clinical infectious diseases 2008, 46, (3), 327–360. - PubMed
-
- Hayes D Jr.; Murphy BS; Lynch JE; Feola DJ Aerosolized amphotericin for the treatment of allergic bronchopulmonary aspergillosis. Pediatric pulmonology 2010, 45, (11), 1145–8. - PubMed
-
- Hayes D Jr.; Mansour HM Improved outcomes of patients with end-stage cystic fibrosis requiring invasive mechanical ventilation for acute respiratory failure. Lung 2011, 189, (5), 409–15. - PubMed
-
- Soubani AO; Chandrasekar PH The clinical spectrum of pulmonary aspergillosis. CHEST Journal 2002, 121, (6), 1988–1999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

