Cyclin B3 activates the Anaphase-Promoting Complex/Cyclosome in meiosis and mitosis
- PMID: 33137813
- PMCID: PMC7660922
- DOI: 10.1371/journal.pgen.1009184
Cyclin B3 activates the Anaphase-Promoting Complex/Cyclosome in meiosis and mitosis
Abstract
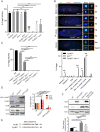
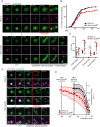
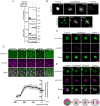
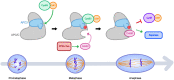
In mitosis and meiosis, chromosome segregation is triggered by the Anaphase-Promoting Complex/Cyclosome (APC/C), a multi-subunit ubiquitin ligase that targets proteins for degradation, leading to the separation of chromatids. APC/C activation requires phosphorylation of its APC3 and APC1 subunits, which allows the APC/C to bind its co-activator Cdc20. The identity of the kinase(s) responsible for APC/C activation in vivo is unclear. Cyclin B3 (CycB3) is an activator of the Cyclin-Dependent Kinase 1 (Cdk1) that is required for meiotic anaphase in flies, worms and vertebrates. It has been hypothesized that CycB3-Cdk1 may be responsible for APC/C activation in meiosis but this remains to be determined. Using Drosophila, we found that mutations in CycB3 genetically enhance mutations in tws, which encodes the B55 regulatory subunit of Protein Phosphatase 2A (PP2A) known to promote mitotic exit. Females heterozygous for CycB3 and tws loss-of-function alleles lay embryos that arrest in mitotic metaphase in a maternal effect, indicating that CycB3 promotes anaphase in mitosis in addition to meiosis. This metaphase arrest is not due to the Spindle Assembly Checkpoint (SAC) because mutation of mad2 that inactivates the SAC does not rescue the development of embryos from CycB3-/+, tws-/+ females. Moreover, we found that CycB3 promotes APC/C activity and anaphase in cells in culture. We show that CycB3 physically associates with the APC/C, is required for phosphorylation of APC3, and promotes APC/C association with its Cdc20 co-activators Fizzy and Cortex. Our results strongly suggest that CycB3-Cdk1 directly activates the APC/C to promote anaphase in both meiosis and mitosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Mitotic phosphatase activity is required for MCC maintenance during the spindle checkpoint.Cell Cycle. 2016;15(2):225-33. doi: 10.1080/15384101.2015.1121331. Cell Cycle. 2016. PMID: 26652909 Free PMC article.
-
Mutations in mákos, a Drosophila gene encoding the Cdc27 subunit of the anaphase promoting complex, enhance centrosomal defects in polo and are suppressed by mutations in twins/aar, which encodes a regulatory subunit of PP2A.J Cell Sci. 2003 Oct 15;116(Pt 20):4147-58. doi: 10.1242/jcs.00722. Epub 2003 Sep 2. J Cell Sci. 2003. PMID: 12953067
-
Cdk1 phosphorylation sites on Cdc27 are required for correct chromosomal localisation and APC/C function in syncytial Drosophila embryos.J Cell Sci. 2007 Jun 15;120(Pt 12):1990-7. doi: 10.1242/jcs.006833. Epub 2007 May 22. J Cell Sci. 2007. PMID: 17519285 Free PMC article.
-
Mechanisms and regulation of the degradation of cyclin B.Philos Trans R Soc Lond B Biol Sci. 1999 Sep 29;354(1389):1571-5; discussion 1575-6. doi: 10.1098/rstb.1999.0500. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10582242 Free PMC article. Review.
-
Regulation of APC-Cdc20 by the spindle checkpoint.Curr Opin Cell Biol. 2002 Dec;14(6):706-14. doi: 10.1016/s0955-0674(02)00382-4. Curr Opin Cell Biol. 2002. PMID: 12473343 Review.
Cited by
-
ADARp150 counteracts whole genome duplication.Nucleic Acids Res. 2024 Sep 23;52(17):10370-10384. doi: 10.1093/nar/gkae700. Nucleic Acids Res. 2024. PMID: 39189458 Free PMC article.
-
Analysis of ovarian transcriptomes reveals thousands of novel genes in the insect vector Rhodnius prolixus.Sci Rep. 2021 Jan 21;11(1):1918. doi: 10.1038/s41598-021-81387-1. Sci Rep. 2021. PMID: 33479356 Free PMC article.
-
CDC20 in and out of mitosis: a prognostic factor and therapeutic target in hematological malignancies.J Exp Clin Cancer Res. 2022 Apr 30;41(1):159. doi: 10.1186/s13046-022-02363-9. J Exp Clin Cancer Res. 2022. PMID: 35490245 Free PMC article. Review.
-
Convergent Molecular Evolution Associated With Repeated Transitions to Gregarious Larval Behavior in Heliconiini.Mol Biol Evol. 2025 Jul 30;42(8):msaf179. doi: 10.1093/molbev/msaf179. Mol Biol Evol. 2025. PMID: 40729511 Free PMC article.
-
Mitotic progression and dual spindle formation caused by spindle association of de novo-formed microtubule-organizing centers in parthenogenetic embryos of Drosophila ananassae.Genetics. 2023 Feb 9;223(2):iyac178. doi: 10.1093/genetics/iyac178. Genetics. 2023. PMID: 36516293 Free PMC article.
References
-
- Morgan DO. The Cell Cycle: Principles of Control. London: New Science Press; 2007. 297 p.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

