Assessing Myocardial Architecture: The Challenges and Controversies
- PMID: 33137874
- PMCID: PMC7711767
- DOI: 10.3390/jcdd7040047
Assessing Myocardial Architecture: The Challenges and Controversies
Abstract
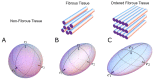
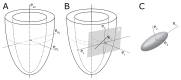
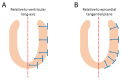
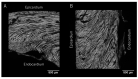
In recent decades, investigators have strived to describe and quantify the orientation of the cardiac myocytes in an attempt to classify their arrangement in healthy and diseased hearts. There are, however, striking differences between the investigations from both a technical and methodological standpoint, thus limiting their comparability and impeding the drawing of appropriate physiological conclusions from the structural assessments. This review aims to elucidate these differences, and to propose guidance to establish methodological consensus in the field. The review outlines the theory behind myocyte orientation analysis, and importantly has identified pronounced differences in the definitions of otherwise widely accepted concepts of myocytic orientation. Based on the findings, recommendations are made for the future design of studies in the field of myocardial morphology. It is emphasised that projection of myocyte orientations, before quantification of their angulation, introduces considerable bias, and that angles should be assessed relative to the epicardial curvature. The transmural orientation of the cardiomyocytes should also not be neglected, as it is an important determinant of cardiac function. Finally, there is considerable disagreement in the literature as to how the orientation of myocardial aggregates should be assessed, but to do so in a mathematically meaningful way, the normal vector of the aggregate plane should be utilised.
Keywords: diffusion tensor imaging; heart; methodology; micro computed tomography; myocardial aggregation; myocyte orientation; review.
Conflict of interest statement
The authors have no conflict of interest.
Figures



References
-
- Omann C., Agger P., Bøgh N., Laustsen C., Ringgaard S., Stephenson R.S., Anderson R.H., Hjortdal V., Smerup M. Resolving the natural myocardial remodelling brought upon by cardiac contraction; a porcine ex-vivo cardiovascular magnetic resonance study of the left and right ventricle. J. Cardiovasc. Magn. Reson. 2019;21:1–19. doi: 10.1186/s12968-019-0547-2. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

