Polymer Ligands Derived from Jute Fiber for Heavy Metal Removal from Electroplating Wastewater
- PMID: 33137923
- PMCID: PMC7692318
- DOI: 10.3390/polym12112521
Polymer Ligands Derived from Jute Fiber for Heavy Metal Removal from Electroplating Wastewater
Abstract
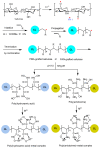
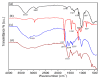
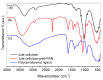
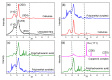
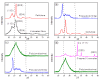
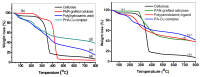
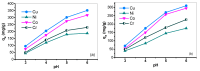
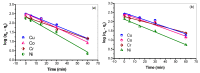
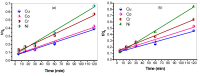
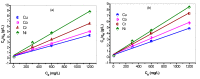
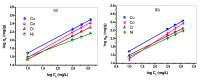
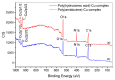
Industrial operations, domestic and agricultural activities worldwide have had major problems with various contaminants caused by environmental pollution. Heavy metal pollution in wastewater also a prominent issue; therefore, a well built and economical treatment technology is demanded for pollution-free wastewater. The present work emphasized pure cellulose extracted from jute fiber and further modification was performed by a free radical grafting reaction, which resulted in poly(methyl acrylate) (PMA)-grafted cellulose and poly(acrylonitrile)-grafted cellulose. Subsequently, poly(hydroxamic acid) and poly(amidoxime) ligands were prepared from the PMA-grafted cellulose and PAN-grafted cellulose, respectively. An adsorption study was performed using the desired ligands with heavy metals such as copper, cobalt, chromium and nickel ions. The binding capacity (qe) with copper ions for poly(hydroxamic acid) is 352 mg g-1 whereas qe for poly(amidoxime) ligand it was exhibited as 310 mg g-1. Other metal ions (chromium, cobalt and nickel) show significance binding properties at pH 6. The Langmuir and Freundlich isotherm study was also performed. The Freundlich isotherm model showed good correlation coefficients for all metal ions, indicating that multiple-layers adsorption was occurred by the polymer ligands. The reusability was evaluated and the adsorbents can be reused for 7 cycles without significant loss of removal performance. Both ligands showed outstanding metals removal capacity from the industrial wastewater as such 98% of copper can be removed from electroplating wastewater and other metals (cobalt, chromium, nickel and lead) can also be removed up to 90%.
Keywords: adsorption; graft copolymer; jute cellulose; poly(amidoxime) ligand; poly(hydroxamic acid); wastewater.
Conflict of interest statement
The authors declared no conflict of interest.
Figures





References
-
- Lakherwal D. Adsorption of Heavy Metals: A Review. Int. J. Environ. Res. Dev. 2014;4:41–48.
-
- Gunatilake S.K. Methods of Removing Heavy Metals for Industrial Wastewater. J. Multidiscip. Eng. Sci. Stud. 2015;1:12–18.
-
- Mnasri-Ghnimi S., Frini-Srasra N. Removal of heavy metals from aqueous solutions by adsorption using single and mixed pillared clays. Appl. Clay Sci. 2019;179:105151. doi: 10.1016/j.clay.2019.105151. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

