Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products
- PMID: 33137942
- PMCID: PMC7692606
- DOI: 10.3390/pharmaceutics12111031
Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products
Abstract
Prodrugs are bioreversible, inactive drug derivatives, which have the ability to convert into a parent drug in the body. In the past, prodrugs were used as a last option; however, nowadays, prodrugs are considered already in the early stages of drug development. Optimal prodrug needs to have effective absorption, distribution, metabolism, and elimination (ADME) features to be chemically stable, to be selective towards the particular site in the body, and to have appropriate safety. Traditional prodrug approach aims to improve physicochemical/biopharmaceutical drug properties; modern prodrugs also include cellular and molecular parameters to accomplish desired drug effect and site-specificity. Here, we present recently investigated prodrugs, their pharmaceutical and clinical advantages, and challenges facing the overall prodrug development. Given examples illustrate that prodrugs can accomplish appropriate solubility, increase permeability, provide site-specific targeting (i.e., to organs, tissues, enzymes, or transporters), overcome rapid drug metabolism, decrease toxicity, or provide better patient compliance, all with the aim to provide optimal drug therapy and outcome. Overall, the prodrug approach is a powerful tool to decrease the time/costs of developing new drug entities and improve overall drug therapy.
Keywords: ProTide; biopharmaceutics; drug absorption; drug delivery; drug targeting; oral administration; prodrugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

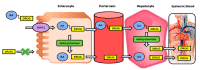
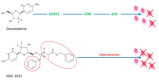
References
-
- Knittel J.J., Zavod R.M. Drug design and relationship of functional groups to pharmacologic activity. In: Lemke T., Williams D.A., Roche V.F., Zito S.W., editors. Foye’s Principles of Medicinal Chemistry. 7th ed. Wolters Kluwer Lippincott Williams and Wilkins; London, UK: 2013. p. 51.
-
- Beig A., Fine-Shamir N., Porat D., Lindley D., Miller J.M., Dahan A. Concomitant solubility-permeability increase: Vitamin E TPGS vs. amorphous solid dispersion as oral delivery systems for etoposide. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V. 2017;121:97–103. doi: 10.1016/j.ejpb.2017.09.012. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

