Quantifying PD-L1 Expression to Monitor Immune Checkpoint Therapy: Opportunities and Challenges
- PMID: 33137949
- PMCID: PMC7692040
- DOI: 10.3390/cancers12113173
Quantifying PD-L1 Expression to Monitor Immune Checkpoint Therapy: Opportunities and Challenges
Abstract
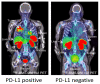
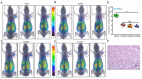
Therapeutics targeting programmed death ligand 1 (PD-L1) protein and its receptor PD-1 are now dominant players in restoring anti-tumor immune responses. PD-L1 detection by immunohistochemistry (IHC) is emerging as a reproducible biomarker for guiding patient stratification for those therapies in some cancers. However, PD-L1 expression in the tumor microenvironment is highly complex. It is upregulated by aberrant genetic alterations, and is highly regulated at the transcriptional, posttranscriptional, and protein levels. Thus, PD-L1 IHC is inadequate to fully understand the relevance of PD-L1 levels in the whole body and their dynamics to improve therapeutic outcomes. Imaging technologies could potentially assist in meeting that need. Early clinical investigations show promising results in quantifying PD-L1 expression in the whole body by positron emission tomography (PET). Within this context, this review summarizes advancements in regulation of PD-L1 expression and imaging agents, and in PD-L1 PET for drug development, and discusses opportunities and challenges presented by these innovations for guiding immune checkpoint therapy (ICT).
Keywords: PET imaging; immune checkpoints; immuno-Oncology; interferon-γ signaling; tumor microenvironment; tumor mutational burden.
Conflict of interest statement
S.N. is a co-inventor on a pending U.S. patent covering WL12 and as such is entitled to a portion of any licensing fees and royalties generated by this technology. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. S.N. owns equity in and serves as a consultant to Precision Molecular, Inc.
Figures


Similar articles
-
Application of molecular imaging in immune checkpoints therapy: From response assessment to prognosis prediction.Crit Rev Oncol Hematol. 2022 Aug;176:103746. doi: 10.1016/j.critrevonc.2022.103746. Epub 2022 Jun 22. Crit Rev Oncol Hematol. 2022. PMID: 35752425 Review.
-
Development of Radiotracers for Imaging of the PD-1/PD-L1 Axis.Pharmaceuticals (Basel). 2022 Jun 14;15(6):747. doi: 10.3390/ph15060747. Pharmaceuticals (Basel). 2022. PMID: 35745666 Free PMC article. Review.
-
Role of tumor microenvironment in the regulation of PD-L1: A novel role in resistance to cancer immunotherapy.J Cell Physiol. 2020 Oct;235(10):6496-6506. doi: 10.1002/jcp.29671. Epub 2020 Apr 2. J Cell Physiol. 2020. PMID: 32239707 Review.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
Practical Immuno-PET Radiotracer Design Considerations for Human Immune Checkpoint Imaging.J Nucl Med. 2017 Apr;58(4):538-546. doi: 10.2967/jnumed.116.177659. Epub 2016 Dec 15. J Nucl Med. 2017. PMID: 27980047 Free PMC article.
Cited by
-
The Prognostic and Predictive Value of Human Gastrointestinal Microbiome and Exosomal mRNA Expression of PD-L1 and IFNγ for Immune Checkpoint Inhibitors Response in Metastatic Melanoma Patients: PROTOCOL TRIAL.Biomedicines. 2023 Jul 18;11(7):2016. doi: 10.3390/biomedicines11072016. Biomedicines. 2023. PMID: 37509655 Free PMC article.
-
Evaluation of single domain antibodies as nuclear tracers for imaging of the immune checkpoint receptor human lymphocyte activation gene-3 in cancer.EJNMMI Res. 2021 Nov 2;11(1):115. doi: 10.1186/s13550-021-00857-9. EJNMMI Res. 2021. PMID: 34727262 Free PMC article.
-
PD-1/PD-L1 blockade therapy with atezolizumab: a new paradigm in the treatment of non-small cell lung cancer (NSCLC).Discov Oncol. 2025 Mar 26;16(1):407. doi: 10.1007/s12672-025-02076-3. Discov Oncol. 2025. PMID: 40140170 Free PMC article. Review.
-
Novel Dual-Mode NIR-II/MRI Nanoprobe Targeting PD-L1 Accurately Evaluates the Efficacy of Immunotherapy for Triple-Negative Breast Cancer.Int J Nanomedicine. 2023 Sep 8;18:5141-5157. doi: 10.2147/IJN.S417944. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37705867 Free PMC article.
-
The Expression of Programmed Death-Ligand 1 on Immune Cells Is Related to a Better Prognosis in Biliary Tract Cancer.Gut Liver. 2023 Nov 15;17(6):933-941. doi: 10.5009/gnl220206. Epub 2022 Dec 13. Gut Liver. 2023. PMID: 36510775 Free PMC article.
References
-
- Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Sosman J.A., Atkins M.B., Leming P.D., et al. Five-Year Survival and Correlates Among Patients with Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated with Nivolumab. JAMA Oncol. 2019 doi: 10.1001/jamaoncol.2019.2187. - DOI - PMC - PubMed
-
- Taube J.M., Anders R.A., Young G.D., Xu H., Sharma R., McMiller T.L., Chen S., Klein A.P., Pardoll D.M., Topalian S.L., et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

