The Platelet Fraction Is a Novel Reservoir to Detect Lyme Borrelia in Blood
- PMID: 33137967
- PMCID: PMC7694117
- DOI: 10.3390/biology9110366
The Platelet Fraction Is a Novel Reservoir to Detect Lyme Borrelia in Blood
Abstract
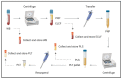
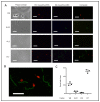
Serological diagnosis of Lyme disease suffers from considerable limitations. Yet, the technique cannot currently be replaced by direct detection methods, such as bacterial culture or molecular analysis, due to their inadequate sensitivity. The low bacterial burden in vasculature and lack of consensus around blood-based isolation of the causative pathogen, Borrelia burgdorferi, are central to this challenge. We therefore addressed methodological optimization of Borrelia recovery from blood, first by analyzing existing protocols, and then by using experimentally infected human blood to identify the processing conditions and fractions that increase Borrelia yield. In this proof-of-concept study, we now report two opportunities to improve recovery and detection of Borrelia from clinical samples. To enhance pathogen viability and cultivability during whole blood collection, citrate anticoagulant is superior to more commonly used EDTA. Despite the widespread reliance on serum and plasma as analytes, we found that the platelet fraction of blood concentrates Borrelia, providing an enriched resource for direct pathogen detection by microscopy, laboratory culture, Western blot, and PCR. The potential for platelets to serve as a reservoir for Borrelia and its diagnostic targets may transform direct clinical detection of this pathogen.
Keywords: Borrelia; EDTA; Lyme disease; OspA; blood processing; citrate; culture; diagnosis; molecular detection; platelets.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Godar D.A., Laniosz V., Wetter D.A. Lyme Disease Update for the General Dermatologist. Am. J. Clin. Dermatol. 2015;16:5–18. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

