The Emerging and Diverse Roles of Bis(monoacylglycero) Phosphate Lipids in Cellular Physiology and Disease
- PMID: 33137979
- PMCID: PMC7663174
- DOI: 10.3390/ijms21218067
The Emerging and Diverse Roles of Bis(monoacylglycero) Phosphate Lipids in Cellular Physiology and Disease
Abstract
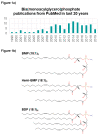
Although understudied relative to many phospholipids, accumulating evidence suggests that bis(monoacylglycero)phosphate (BMP) is an important class of regulatory lipid that plays key roles in lysosomal integrity and function. BMPs are rare in most mammalian tissues, comprising only a few percent of total cellular lipid content, but are elevated in cell types such as macrophages that rely heavily on lysosomal function. BMPs are markedly enriched in endosomal and lysosomal vesicles compared to other organelles and membranous structures, and their unique sn-1:sn-1' stereoconfiguration may confer stability within the hydrolytic lysosomal environment. BMP-enriched vesicles serve in endosomal-lysosomal trafficking and function as docking structures for the activation of lysosomal hydrolytic enzymes, notably those involved in the catabolic breakdown of sphingolipids. BMP levels are dysregulated in lysosomal storage disorders, phospholipidosis, metabolic diseases, liver and kidney diseases and neurodegenerative disorders. However, whether BMP alteration is a mediator or simply a marker of pathological states is unclear. Likewise, although BMP acyl chain composition may be altered with disease states, the functional significance of specific BMP species remains to be resolved. Newly developed tools for untargeted lipidomic analysis, together with a deeper understanding of enzymes mediating BMP synthesis and degradation, will help shed further light on the functional significance of BMPs in cellular physiology and pathology.
Keywords: bis(monoacylglycero)phosphate; lipidomics; lysobisphophatidic acid; lysosome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gray D.R.B.G.M. The isolation and characterisation of phosphatidylglycerol and a structural isomer from pig lung. Chem. Phys. Lipids. 1967;1:254–263. doi: 10.1016/0009-3084(67)90032-1. - DOI
-
- Record M., Amara S., Subra C., Jiang G., Prestwich G.D., Ferrato F., Carriere F. Bis (monoacylglycero) phosphate interfacial properties and lipolysis by pancreatic lipase-related protein 2, an enzyme present in THP-1 human monocytes. Biochim. Biophys. Acta. 2011;1811:419–430. doi: 10.1016/j.bbalip.2011.04.008. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

