Adjunctive Use of Phage Sb-1 in Antibiotics Enhances Inhibitory Biofilm Growth Activity versus Rifampin-Resistant Staphylococcus aureus Strains
- PMID: 33138034
- PMCID: PMC7692760
- DOI: 10.3390/antibiotics9110749
Adjunctive Use of Phage Sb-1 in Antibiotics Enhances Inhibitory Biofilm Growth Activity versus Rifampin-Resistant Staphylococcus aureus Strains
Abstract
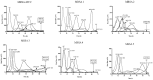
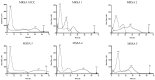
Effective antimicrobials are crucial for managing Staphylococcus aureus implant-associated bone infections (IABIs), particularly for infections due to rifampin-resistant S. aureus (RRSA). Failure to remove the implant results in persistent infection; thus, prolonged suppressive antibiotic therapy may be a reasonable alternative. However, a high incidence of adverse events can necessitate the discontinuation of therapy. In this scenario, commercial Staphylococcal bacteriophage Sb-1 combined with antibiotics is an option, showing a promising synergistic activity to facilitate the treatment of biofilm infections. Therefore, we evaluated the efficacy of the inhibitory activity of five antibiotics (doxycycline, levofloxacin, clindamycin, linezolid, and rifampin) alone or combined with phage Sb-1 (106 PFU/mL) in a simultaneous and staggered manner, to combat five clinical RRSA strains and the laboratory strain MRSA ATCC 43300 in 72 h by isothermal microcalorimetry. The synergistic effects were observed when phage Sb-1 (106 PFU/mL) combined with antibiotics had at least 2 log-reduction lower concentrations, represented by a fractional biofilm inhibitory concentration (FBIC) of <0.25. Among the antibiotics that we tested, the synergistic effect of all six strains was achieved in phage/doxycycline and phage/linezolid combinations in a staggered manner, whereas a distinctly noticeable improvement in inhibitory activity was observed in the phage/doxycycline combination with a low concentration of doxycycline. Moreover, phage/levofloxacin and phage/clindamycin combinations also showed a synergistic inhibitory effect against five strains and four strains, respectively. Interestingly, the synergistic inhibitory activity was also observed in the doxycycline-resistant and levofloxacin-resistant profile strains. However, no inhibitory activity was observed for all of the combinations in a simultaneous manner, as well as for the phage/rifampin combination in a staggered manner. These results have implications for alternative, combined, and prolonged suppressive antimicrobial treatment approaches.
Keywords: antibiotic-phage combination; biofilm infection; rifampin-resistant Staphylococcus aureus; suppression therapy; synergism.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Moriarty T.F., Kuehl R., Coenye T., Metsemakers W.J., Morgenstern M., Schwarz E.M., Riool M., Zaat S.A.J., Khana N., Kates S.L., et al. Orthopaedic device-related infection: Current and future interventions for improved prevention and treatment. Efort Open Rev. 2016;1:89–99. doi: 10.1302/2058-5241.1.000037. - DOI - PMC - PubMed
-
- Kolenda C., Josse J., Medina M., Fevre C., Lustig S., Ferry T., Laurent F. Evaluation of the Activity of a Combination of Three Bacteriophages Alone or in Association with Antibiotics on Staphylococcus aureus Embedded in Biofilm or Internalized in Osteoblasts. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.02231-19. - DOI - PMC - PubMed
-
- Murillo O., Pachón M.E., Euba G., Verdaguer R., Tubau F., Cabellos C., Cabo J., Gudiol F., Ariza J. Antagonistic effect of rifampin on the efficacy of high-dose levofloxacin in staphylococcal experimental foreign-body infection. Antimicrob. Agents Chemother. 2008;52:3681–3686. doi: 10.1128/AAC.00458-08. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

