Antihypertensive Effects of Gynura divaricata (L.) DC in Rats with Renovascular Hypertension
- PMID: 33138042
- PMCID: PMC7692656
- DOI: 10.3390/nu12113321
Antihypertensive Effects of Gynura divaricata (L.) DC in Rats with Renovascular Hypertension
Abstract
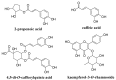
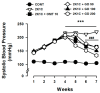
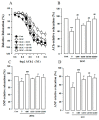
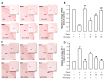
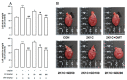
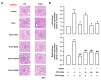
Gynura divaricata (L.) DC (Compositae) (GD) could be found in various parts of Asia. It has been used as a traditional medicine to treat diabetes, high blood pressure, and other diseases, but its effects have not yet been scientifically confirmed. Therefore, we aimed at determining whether GD could affect renal function regulation, blood pressure, and the renin-angiotensin-aldosterone system (RAAS). Cardio-renal syndrome (CRS) is a disease caused by the interaction between the kidney and the cardiovascular system, where the acute or chronic dysfunction in one organ might induce acute or chronic dysfunction of the other. This study investigated whether GD could improve cardio-renal mutual in CRS type 4 model animals, two-kidney one-clip (2K1C) renal hypertensive rats. The experiments were performed on the following six experimental groups: control rats (CONT); 2K1C rats (negative control); OMT (Olmetec, 10 mg/kg/day)-treated 2K1C rats (positive control); and 2K1C rats treated with GD extracts in three different doses (50, 100, and 200 mg/kg/day) for three weeks by oral intake. Each group consisted of 10 rats. We measured the systolic blood pressure weekly using the tail-cuff method. Urine was also individually collected from the metabolic cage to investigate the effect of GD on the kidney function, monitoring urine volume, electrolyte, osmotic pressure, and creatinine levels from the collected urine. We observed that kidney weight and urine volume, which would both display typically increased values in non-treated 2K1C animals, significantly decreased following the GD treatment (###p < 0.001 vs. 2K1C). Osmolality and electrolytes were measured in the urine to determine how renal excretory function, which is reduced in 2K1C rats, could be affected. We found that the GD treatment improved renal excretory function. Moreover, using periodic acid-Schiff staining, we confirmed that the GD treatment significantly reduced fibrosis, which is typically increased in 2K1C rats. Thus, we confirmed that the GD treatment improved kidney function in 2K1C rats. Meanwhile, we conducted blood pressure and vascular relaxation studies to determine if the GD treatment could improve cardiovascular function in 2K1C rats. The heart weight percentages of the left atrium and ventricle were significantly lower in GD-treated 2K1C rats than in non-treated 2K1C rats. These results showed that GD treatment reduced cardiac hypertrophy in 2K1C rats. Furthermore, the acetylcholine-, sodium nitroprusside-, and atrial natriuretic peptide-mediated reduction of vasodilation in 2K1C rat aortic rings was also ameliorated by GD treatment (GD 200 mg/kg/day; p < 0.01, p < 0.05, and p < 0.05 vs. 2K1C for vasodilation percentage in case of each compound). The mRNA expression in the 2K1C rat heart tissue showed that the GD treatment reduced brain-type natriuretic peptide and troponin T levels (p < 0.001 and p < 0.001 vs. 2K1C). In conclusion, this study showed that GD improved the cardiovascular and renal dysfunction observed in an innovative hypertension model, highlighting the potential of GD as a therapeutic agent for hypertension. These findings indicate that GD shows beneficial effects against high blood pressure by modulating the RAAS in the cardio-renal syndrome. Thus, it should be considered an effective traditional medicine in hypertension treatment.
Keywords: Gynura divaricata (L.) DC; cardio-renal syndrome; renin-angiotensin-aldosterone system (RAAS); renovascular hypertension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
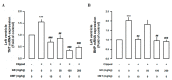

Similar articles
-
Aldosterone Contributes to Sympathoexcitation in Renovascular Hypertension.Am J Hypertens. 2015 Sep;28(9):1083-90. doi: 10.1093/ajh/hpu300. Epub 2015 Jan 26. Am J Hypertens. 2015. PMID: 25628418
-
Renal sympathetic denervation attenuates hypertension and vascular remodeling in renovascular hypertensive rats.J Appl Physiol (1985). 2017 Jan 1;122(1):121-129. doi: 10.1152/japplphysiol.01019.2015. Epub 2016 Oct 14. J Appl Physiol (1985). 2017. PMID: 27742806
-
Diuretic herb Gomphrena celosioides Mart. (Amaranthaceae) promotes sustained arterial pressure reduction and protection from cardiac remodeling on rats with renovascular hypertension.J Ethnopharmacol. 2018 Oct 5;224:126-133. doi: 10.1016/j.jep.2018.05.036. Epub 2018 May 26. J Ethnopharmacol. 2018. PMID: 29842964
-
Novel therapeutic targets for cardiorenal syndrome.Drug Discov Today. 2025 Jan;30(1):104285. doi: 10.1016/j.drudis.2024.104285. Epub 2025 Jan 4. Drug Discov Today. 2025. PMID: 39761847 Review.
-
Antihypertensive drug treatment: are we ready for the future?J Hypertens. 2025 Jul 1;43(7):1099-1107. doi: 10.1097/HJH.0000000000004019. Epub 2025 Apr 1. J Hypertens. 2025. PMID: 40167023 Review.
Cited by
-
Peperomia pellucida (L.) Kunth as an angiotensin-converting enzyme inhibitor in two-kidney, one-clip Goldblatt hypertensive rats.Saudi J Biol Sci. 2021 Nov;28(11):6191-6197. doi: 10.1016/j.sjbs.2021.06.075. Epub 2021 Jun 30. Saudi J Biol Sci. 2021. PMID: 34759740 Free PMC article.
-
Nobiletin resolves left ventricular and renal changes in 2K-1C hypertensive rats.Sci Rep. 2022 Jun 3;12(1):9289. doi: 10.1038/s41598-022-13513-6. Sci Rep. 2022. PMID: 35662276 Free PMC article.
-
Editorial: Nutrition Management for Chronic Kidney Disease.Nutrients. 2020 Dec 17;12(12):3852. doi: 10.3390/nu12123852. Nutrients. 2020. PMID: 33348550 Free PMC article.
-
Evaluation of Xanthine Oxidase Inhibitors Febuxostat and Allopurinol on Kidney Dysfunction and Histological Damage in Two-Kidney, One-Clip (2K1C) Rats.Scientifica (Cairo). 2025 Jan 22;2025:7932075. doi: 10.1155/sci5/7932075. eCollection 2025. Scientifica (Cairo). 2025. PMID: 39886537 Free PMC article.
-
Quantification of Usaramine and its N-Oxide Metabolite in Rat Plasma Using Liquid Chromatography-Tandem Mass Spectrometry.J Anal Toxicol. 2022 May 20;46(5):512-518. doi: 10.1093/jat/bkab060. J Anal Toxicol. 2022. PMID: 34086913 Free PMC article.
References
-
- Robinson A.T., Babcock M.C., Watso J.C., Brian M.S., Migdal K.U., Wenner M.M., Farquhar W.B. Relation between resting sympathetic outflow and vasoconstrictor responses to sympathetic nerve bursts: Sex differences in healthy young adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019;316:463–471. doi: 10.1152/ajpregu.00305.2018. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

