Plasma Predictive Features in Treating EGFR-Mutated Non-Small Cell Lung Cancer
- PMID: 33138052
- PMCID: PMC7692448
- DOI: 10.3390/cancers12113179
Plasma Predictive Features in Treating EGFR-Mutated Non-Small Cell Lung Cancer
Abstract
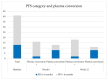
Although epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) are the preferred treatment for patients with EGFR-mutated non-small cell lung cancer (NSCLC), not all patients benefit. We therefore explored the impact of the presence of mutations found in cell-free DNA (cfDNA) and TKI plasma concentrations during treatment on progression-free survival (PFS). In the prospective START-TKI study blood samples from 41 patients with EGFR-mutated NSCLC treated with EGFR-TKIs were available. Next generation sequencing (NGS) on cfDNA was performed, and plasma TKI concentrations were measured. Patients without complete plasma conversion of EGFR mutation at week 6 had a significantly shorter PFS (5.5 vs. 17.0 months, p = 0.002) and OS (14.0 vs. 25.5 months, p = 0.003) compared to patients with plasma conversion. In thirteen (second line) osimertinib-treated patients with a (plasma or tissue) concomitant TP53 mutation at baseline, PFS was significantly shorter compared to six wild-type cases; 8.8 vs. 18.8 months, p = 0.017. Erlotinib Cmean decrease of ≥10% in the second tertile of treatment was also associated with a significantly shorter PFS; 8.9 vs. 23.6 months, p = 0.037. We obtained evidence that absence of plasma loss of the primary EGFR mutation, isolated plasma p.T790M loss after six weeks, baseline concomitant TP53 mutations, and erlotinib Cmean decrease during treatment are probably related to worse outcome.
Keywords: EGFR; NSCLC; T790M mutation; TKI; TP53 mutation; cfDNA; pharmacokinetics; plasma conversion.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. C.S. reports financial activities outside the submitted work: advisory board Boehringer Ingelheim, travel and symposia expenses from Roche, Lilly. C.L. reports financial activities outside the submitted work: BMS, Boeringer Ingelheim, MSD, Roche, Abbvie, AstraZeneca. S.K. reports financial activities outside the submitted work: lecture for Roche. W.D. reports financial activities outside the submitted work: Speakers and advisory honoraria from Roche, Bristol-Myers Squibb, Amgen, Bayer, AstraZeneca, Novartis. R.M. reports financial activities outside the submitted work: grants from The Dutch Cancer Society (KWF), Astellas, Bayer, Boehringer-Ingelheim, Cristal Therapeutics, Pamgene (and other), Pfizer, Prostakan, Novartis (and personal fees), Roche, Servier (and personal fees). J.A. reports financial activities outside the submitted work: MSD, BMS, Boehringer Ingelheim, Amphera, Eli-Lilly, Takeda, Bayer, Roche, AstraZeneca. Intellectual property: licensed patent on allogenic tumor cell lysate, pending patent on combination imunotherapy in cancer and biomarker for immunotherapy. H.D. reports grants, personal fees and non-financial support from AstraZeneca with regard to the Work Under Consideration for Publication and personal fees from AbbVie, Janssen, Pfizer, Lilly, PGDx, MSD outside the submitted work. A.D. reports financial activities outside the submitted work: Attended advisory boards and/or provided lectures for: Roche, Eli Lilly, Boehringer Ingelheim, Astra Zeneca, Pfizer, BMS, Amgen, Novartis, MSD, Takeda, Pharmamar. Received research support from BMS, AbbVie, Amgen. M.V., M.P., P.A., M.P., J.T., D.Y., E.O., and R.S. declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

