Marine-Derived Penicillium purpurogenum Reduces Tumor Size and Ameliorates Inflammation in an Erlich Mice Model
- PMID: 33138062
- PMCID: PMC7694122
- DOI: 10.3390/md18110541
Marine-Derived Penicillium purpurogenum Reduces Tumor Size and Ameliorates Inflammation in an Erlich Mice Model
Abstract
Background: This study addresses the antitumoral properties of Penicillium purpurogenum isolated from a polluted lagoon in Northeastern Brazil.
Methods: Ethyl Acetate Extracellular Extract (EAE) was used. The metabolites were studied using direct infusion mass spectrometry. The solid Ehrlich tumor model was used for antitumor activity. Female Swiss mice were divided into groups (n = 10/group) as follows: The negative control (CTL-), treated with a phosphate buffered solution; the positive control (CTL+), treated with cyclophosphamide (25 mg/kg); extract treatments at doses of 4, 20, and 100 mg/kg; animals without tumors or treatments (Sham); and animals without tumors treated with an intermediate dose (EAE20). All treatments were performed intraperitoneally, daily, for 15 days. Subsequently, the animals were euthanized, and the tumor, lymphoid organs, and serum were used for immunological, histological, and biochemical parameter evaluations.
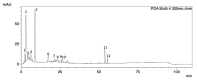
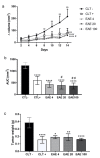
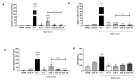
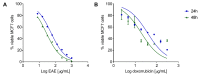
Results: The extract was rich in meroterpenoids. All doses significantly reduced tumor size, and the 20 and 100 mg/kg doses reduced tumor-associated inflammation and tumor necrosis. The extract also reduced the cellular infiltration of lymphoid organs and circulating TNF-α levels. The extract did not induce weight loss or renal and hepatic toxic changes.
Conclusions: These results indicate that P. purpurogenum exhibits immunomodulatory and antitumor properties in vivo. Thus, fungal fermentation is a valid biotechnological approach to the production of antitumor agents.
Keywords: Ehlich’s tumor; P. purpurogenum; antitumor; inflammation; meroterpenoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Marine Natural Products as Anticancer Agents.Mar Drugs. 2021 Aug 4;19(8):447. doi: 10.3390/md19080447. Mar Drugs. 2021. PMID: 34436286 Free PMC article.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

