Drug Repurposing for Triple-Negative Breast Cancer
- PMID: 33138097
- PMCID: PMC7711505
- DOI: 10.3390/jpm10040200
Drug Repurposing for Triple-Negative Breast Cancer
Abstract
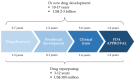
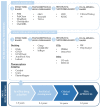
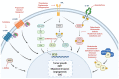
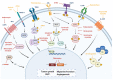
Triple-negative breast cancer (TNBC) is the most aggressive type of breast cancer which presents a high rate of relapse, metastasis, and mortality. Nowadays, the absence of approved specific targeted therapies to eradicate TNBC remains one of the main challenges in clinical practice. Drug discovery is a long and costly process that can be dramatically improved by drug repurposing, which identifies new uses for existing drugs, both approved and investigational. Drug repositioning benefits from improvements in computational methods related to chemoinformatics, genomics, and systems biology. To the best of our knowledge, we propose a novel and inclusive classification of those approaches whereby drug repurposing can be achieved in silico: structure-based, transcriptional signatures-based, biological networks-based, and data-mining-based drug repositioning. This review specially emphasizes the most relevant research, both at preclinical and clinical settings, aimed at repurposing pre-existing drugs to treat TNBC on the basis of molecular mechanisms and signaling pathways such as androgen receptor, adrenergic receptor, STAT3, nitric oxide synthase, or AXL. Finally, because of the ability and relevance of cancer stem cells (CSCs) to drive tumor aggressiveness and poor clinical outcome, we also focus on those molecules repurposed to specifically target this cell population to tackle recurrence and metastases associated with the progression of TNBC.
Keywords: cancer stem cells; clinical trials; computational methods; drug repurposing; personalized medicine; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

