The Design of Anionic Surfactant-Based Amino-Functionalized Mesoporous Silica Nanoparticles and their Application in Transdermal Drug Delivery
- PMID: 33138139
- PMCID: PMC7693828
- DOI: 10.3390/pharmaceutics12111035
The Design of Anionic Surfactant-Based Amino-Functionalized Mesoporous Silica Nanoparticles and their Application in Transdermal Drug Delivery
Abstract
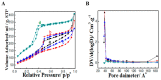
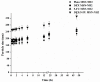
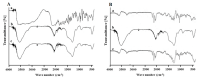
Melanoma remains the most lethal form of skin cancer and most challenging to treat despite advances in the oncology field. Our work describes the utilization of nanotechnology to target melanoma locally in an attempt to provide an advanced and efficient quality of therapy. Amino-functionalized mesoporous silica nanoparticles (MSN-NH2) were developed in situ through the utilization of anionic surfactant and different volumes of 3-aminopropyltriethoxysilane (APTES) as a co-structure directing agent (CSDA). Prepared particles were characterized for their morphology, particles size, 5-flurouracol (5-FU) and dexamethasone (DEX) loading capacity and release, skin penetration, and cytotoxicity in vitro in HT-144 melanoma cells. Results of transmission electron microscopy (TEM) and nitrogen adsorption-desorption isotherm showed that using different volumes of APTES during the functionalization process had an impact on the internal and external morphology of the particles, as well as particle size. However, changing the volume of APTES did not affect the diameter of formed mesochannels, which was about 4 nm. MSN-NH2 showed a relatively high loading capacity of 5-FU (12.6 ± 5.5) and DEX (44.72 ± 4.21) when using drug: MSN-NH2 ratios of 5:1 for both drugs. The release profile showed that around 83% of 5-FU and 21% of DEX were released over 48 h in pH 7.4. The skin permeability study revealed that enhancement ratio of 5-Fu and DEX using MSN-NH2 were 4.67 and 5.68, respectively, relative to their free drugs counterparts. In addition, the accumulation of drugs in skin layers where melanoma cells usually reside were enhanced approximately 10 times with 5-FU and 5 times with DEX when delivering drugs using MSN-NH2 compared to control. MSN-NH2 alone was nontoxic to melanoma cells when incubated for 48 h in the range of 0 to 468 µg/mL. The combination of 5-FU MSN-NH2 and DEX MSN-NH2 showed significant increase in toxicity compared to their free dug counterparts and exhibited a synergetic effect as well as the ability to circumvent DEX induced 5-FU resistance in melanoma cells.
Keywords: 5-flurouracil; amino functionalized; dexamethasone; melanoma; mesoporous silica nanoparticles; transdermal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Matthews N.H., Li W.Q., Qureshi A.A., Weinstock M.A., Cho E. Epidemiology of Melanoma. In: Ward W.H., Farma J.M., editors. Cutaneous Melanoma: Etiology and Therapy. Exon Publications; Brisbane, Australia: 2017. - PubMed
-
- Laikova K.V., Oberemok V.V., Krasnodubets A.M., Shumskykh M.N., Useinov R.Z., Novikov I.A., Temirova Z.Z., Gorlov M.V., Shved N.A., Kumeiko V.V., et al. Advances in the Understanding of Skin Cancer: Ultraviolet Radiation, Mutations, and Antisense Oligonucleotides as Anticancer Drugs. Molcules. 2019;24:1516. doi: 10.3390/molecules24081516. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

