Overexpression of α-Synuclein by Oligodendrocytes in Transgenic Mice Does Not Recapitulate the Fibrillar Aggregation Seen in Multiple System Atrophy
- PMID: 33138150
- PMCID: PMC7693764
- DOI: 10.3390/cells9112371
Overexpression of α-Synuclein by Oligodendrocytes in Transgenic Mice Does Not Recapitulate the Fibrillar Aggregation Seen in Multiple System Atrophy
Abstract
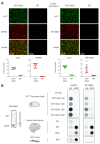
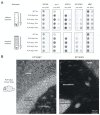
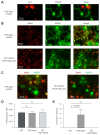
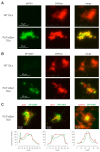
The synucleinopathy underlying multiple system atrophy (MSA) is characterized by the presence of abundant amyloid inclusions containing fibrillar α-synuclein (α-syn) aggregates in the brains of the patients and is associated with an extensive neurodegeneration. In contrast to Parkinson's disease (PD) where the pathological α-syn aggregates are almost exclusively neuronal, the α-syn inclusions in MSA are principally observed in oligodendrocytes (OLs) where they form glial cytoplasmic inclusions (GCIs). This is intriguing because differentiated OLs express low levels of α-syn, yet pathogenic amyloid α-syn seeds require significant amounts of α-syn monomers to feed their fibrillar growth and to eventually cause the buildup of cytopathological inclusions. One of the transgenic mouse models of this disease is based on the targeted overexpression of human α-syn in OLs using the PLP promoter. In these mice, the histopathological images showing a rapid emergence of S129-phosphorylated α-syn inside OLs are considered as equivalent to GCIs. Instead, we report here that they correspond to the accumulation of phosphorylated α-syn monomers/oligomers and not to the appearance of the distinctive fibrillar α-syn aggregates that are present in the brains of MSA or PD patients. In spite of a propensity to co-sediment with myelin sheath contaminants, the phosphorylated forms found in the brains of the transgenic animals are soluble (>80%). In clear contrast, the phosphorylated species present in the brains of MSA and PD patients are insoluble fibrils (>95%). Using primary cultures of OLs from PLP-αSyn mice we observed a variable association of S129-phosphorylated α-syn with the cytoplasmic compartment, the nucleus and with membrane domains suggesting that OLs functionally accommodate the phospho-α-syn deriving from experimental overexpression. Yet and while not taking place spontaneously, fibrillization can be seeded in these primary cultures by challenging the OLs with α-syn preformed fibrils (PFFs). This indicates that a targeted overexpression of α-syn does not model GCIs in mice but that it can provide a basis for seeding aggregation using PFFs. This approach could help establishing a link between α-syn aggregation and the development of a clinical phenotype in these transgenic animals.
Keywords: GCIs; multiple system atrophy; α-synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Oligodendrocytes Prune Axons Containing α-Synuclein Aggregates In Vivo: Lewy Neurites as Precursors of Glial Cytoplasmic Inclusions in Multiple System Atrophy?Biomolecules. 2023 Feb 1;13(2):269. doi: 10.3390/biom13020269. Biomolecules. 2023. PMID: 36830639 Free PMC article.
-
Endogenous oligodendroglial alpha-synuclein and TPPP/p25α orchestrate alpha-synuclein pathology in experimental multiple system atrophy models.Acta Neuropathol. 2019 Sep;138(3):415-441. doi: 10.1007/s00401-019-02014-y. Epub 2019 Apr 22. Acta Neuropathol. 2019. PMID: 31011860 Free PMC article.
-
Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes.EMBO Rep. 2002 Jun;3(6):583-8. doi: 10.1093/embo-reports/kvf109. Epub 2002 May 24. EMBO Rep. 2002. PMID: 12034752 Free PMC article.
-
Is Multiple System Atrophy a Prion-like Disorder?Int J Mol Sci. 2021 Sep 18;22(18):10093. doi: 10.3390/ijms221810093. Int J Mol Sci. 2021. PMID: 34576255 Free PMC article. Review.
-
Insights into the pathogenesis of multiple system atrophy: focus on glial cytoplasmic inclusions.Transl Neurodegener. 2020 Feb 17;9:7. doi: 10.1186/s40035-020-0185-5. eCollection 2020. Transl Neurodegener. 2020. PMID: 32095235 Free PMC article. Review.
Cited by
-
Multiple system atrophy: α-Synuclein strains at the neuron-oligodendrocyte crossroad.Mol Neurodegener. 2022 Nov 26;17(1):77. doi: 10.1186/s13024-022-00579-z. Mol Neurodegener. 2022. PMID: 36435784 Free PMC article. Review.
-
Spreading of Aggregated α-Synuclein in Sagittal Organotypic Mouse Brain Slices.Biomolecules. 2022 Jan 19;12(2):163. doi: 10.3390/biom12020163. Biomolecules. 2022. PMID: 35204664 Free PMC article.
-
Oligodendrocyte-specific overexpression of human alpha-synuclein results in elevated MBP levels and inflammatory responses in TgM83 mice, mimicking the pathological features of multiple system atrophy.Acta Neuropathol Commun. 2025 May 7;13(1):94. doi: 10.1186/s40478-025-02014-y. Acta Neuropathol Commun. 2025. PMID: 40336104 Free PMC article.
-
Immunization targeting diseased proteins in synucleinopathy and tauopathy: insights from clinical trials.Transl Neurodegener. 2025 Jul 1;14(1):33. doi: 10.1186/s40035-025-00490-9. Transl Neurodegener. 2025. PMID: 40588759 Free PMC article. Review.
-
Key Signalling Molecules in Aging and Neurodegeneration.Cells. 2022 Feb 28;11(5):834. doi: 10.3390/cells11050834. Cells. 2022. PMID: 35269456 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

