Plant Derived Natural Products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm Activity and Molecular Mechanisms
- PMID: 33138250
- PMCID: PMC7663672
- DOI: 10.3390/molecules25215024
Plant Derived Natural Products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm Activity and Molecular Mechanisms
Abstract
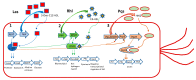
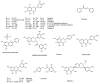
Bacteria are social organisms able to build complex structures, such as biofilms, that are highly organized surface-associated communities of microorganisms, encased within a self- produced extracellular matrix. Biofilm is commonly associated with many health problems since its formation increases resistance to antibiotics and antimicrobial agents, as in the case of Pseudomonas aeruginosa and Staphylococcus aureus, two human pathogens causing major concern. P. aeruginosa is responsible for severe nosocomial infections, the most frequent of which is ventilator-associated pneumonia, while S. aureus causes several problems, like skin infections, septic arthritis, and endocarditis, to name just a few. Literature data suggest that natural products from plants, bacteria, fungi, and marine organisms have proven to be effective as anti-biofilm agents, inhibiting the formation of the polymer matrix, suppressing cell adhesion and attachment, and decreasing the virulence factors' production, thereby blocking the quorum sensing network. Here, we focus on plant derived chemicals, and provide an updated literature review on the anti-biofilm properties of terpenes, flavonoids, alkaloids, and phenolic compounds. Moreover, whenever information is available, we also report the mechanisms of action.
Keywords: Pseudomonas aeruginosa; Staphylococcus aureus; antibiotic-resistance; biofilm; flavonoids; plant-derived natural products; quorum sensing; terpenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

