"FastCheckFLI PPR-like"-A Molecular Tool for the Fast Genome Detection of PPRV and Differential Diagnostic Pathogens
- PMID: 33138260
- PMCID: PMC7694148
- DOI: 10.3390/v12111227
"FastCheckFLI PPR-like"-A Molecular Tool for the Fast Genome Detection of PPRV and Differential Diagnostic Pathogens
Abstract
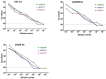
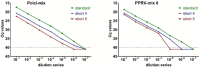
To assist the global eradication of peste des petits ruminants virus (PPRV), a molecular test for the rapid and reliable detection of PPRV was developed which additionally enables the detection of pathogens relevant for differential diagnostics. For this purpose, the necessary time frame of a magnetic bead-based nucleic acid extraction protocol was markedly shortened to 7 min and 13 s. The optimized extraction was run on a BioSprint 15 platform. Furthermore, a high-speed multi-well RT-qPCR for the genome detection of PPRV and additional important pathogens such as Foot-and-mouth disease virus, Parapoxvirus ovis, Goatpox virus, and Mycoplasmacapricolumsubsp.capripneumoniae was established and combined with suitable internal control assays. The here-described qPCR is based on a lyophilized master mix and takes only around 30 to 40 min. Several qPCR cyclers were evaluated regarding their suitability for fast-cycling approaches and for their diagnostic performance in a high-speed RT-qPCR. The final evaluation was conducted on the BioRad CFX96 and also on a portable Liberty16 qPCR cycler. The new molecular test designated as "FastCheckFLI PPR-like", which is based on rapid nucleic acid extraction and high-speed RT-qPCR, delivered reliable results in less than one hour, allowing its use also in a pen-side scenario.
Keywords: Small ruminant morbilli virus; differential diagnosis; fast extraction; high-speed RT-qPCR; molecular pen-side test; peste des petits ruminants virus (PPRV); rapid detection method.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Genetic detection of peste des petits ruminants virus under field conditions: a step forward towards disease eradication.BMC Vet Res. 2017 Jan 25;13(1):34. doi: 10.1186/s12917-016-0940-0. BMC Vet Res. 2017. PMID: 28122564 Free PMC article.
-
Rapid Detection of Peste des Petits Ruminants Virus (PPRV) Nucleic Acid Using a Novel Low-Cost Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) Assay for Future Use in Nascent PPR Eradication Programme.Viruses. 2019 Jul 31;11(8):699. doi: 10.3390/v11080699. Viruses. 2019. PMID: 31370329 Free PMC article.
-
Development of real-time and lateral flow strip reverse transcription recombinase polymerase Amplification assays for rapid detection of peste des petits ruminants virus.Virol J. 2017 Feb 7;14(1):24. doi: 10.1186/s12985-017-0688-6. Virol J. 2017. PMID: 28173845 Free PMC article.
-
Peste des petits ruminants in Africa: a review of currently available molecular epidemiological data, 2020.Arch Virol. 2020 Oct;165(10):2147-2163. doi: 10.1007/s00705-020-04732-1. Epub 2020 Jul 11. Arch Virol. 2020. PMID: 32653984 Free PMC article. Review.
-
Peste Des Petits Ruminants in Benin: Persistence of a Single Virus Genotype in the Country for Over 42 Years.Transbound Emerg Dis. 2017 Aug;64(4):1037-1044. doi: 10.1111/tbed.12471. Epub 2016 Jan 22. Transbound Emerg Dis. 2017. PMID: 26801518 Review.
Cited by
-
Molecular characterization of peste des petits ruminants virus and Mycoplasma capricolum subsp. capripneumoniae in small ruminants in northern Mauritania, 2023.Vet Res Commun. 2024 Dec;48(6):4089-4095. doi: 10.1007/s11259-024-10527-5. Epub 2024 Sep 3. Vet Res Commun. 2024. PMID: 39225972
-
Easy Express Extraction (TripleE)-A Universal, Electricity-Free Nucleic Acid Extraction System for the Lab and the Pen.Microorganisms. 2022 May 23;10(5):1074. doi: 10.3390/microorganisms10051074. Microorganisms. 2022. PMID: 35630515 Free PMC article.
-
Pheno- and genotypic characterization and identification of novel subtypes of Peste des Petits Ruminants virus in domestic and captive wild goats in Northern Iraq.BMC Microbiol. 2021 Dec 7;21(1):334. doi: 10.1186/s12866-021-02372-2. BMC Microbiol. 2021. PMID: 34876012 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

