Vitamin B3-Based Biologically Active Compounds as Inhibitors of Human Cholinesterases
- PMID: 33138280
- PMCID: PMC7663184
- DOI: 10.3390/ijms21218088
Vitamin B3-Based Biologically Active Compounds as Inhibitors of Human Cholinesterases
Abstract
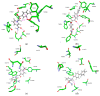
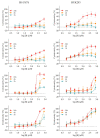
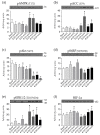
We evaluated the potential of nine vitamin B3 scaffold-based derivatives as acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors, as a starting point for the development of novel drugs for treating disorders with cholinergic neurotransmission-linked pathology. As the results indicate, all compounds reversibly inhibited both enzymes in the micromolar range pointing to the preference of AChE over BChE for binding the tested derivatives. Molecular docking studies revealed the importance of interactions with AChE active site residues Tyr337 and Tyr124, which dictated most of the observed differences. The most potent inhibitor of both enzymes with Ki of 4 μM for AChE and 8 μM for BChE was the nicotinamide derivative 1-(4'-phenylphenacyl)-3-carbamoylpyridinium bromide. Such a result places it within the range of several currently studied novel cholinesterase inhibitors. Cytotoxicity profiling did not classify this compound as highly toxic, but the induced effects on cells should not be neglected in any future detailed studies and when considering this scaffold for drug development.
Keywords: AChE; Alzheimer’s; BChE; cytotoxicity; neurodegenerative; nicotinamide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Flavonols and 4-thioflavonols as potential acetylcholinesterase and butyrylcholinesterase inhibitors: Synthesis, structure-activity relationship and molecular docking studies.Bioorg Chem. 2019 Oct;91:103124. doi: 10.1016/j.bioorg.2019.103124. Epub 2019 Jul 12. Bioorg Chem. 2019. PMID: 31319297
-
Syntheses, cholinesterases inhibition, and molecular docking studies of pyrido[2,3-b]pyrazine derivatives.Chem Biol Drug Des. 2015 Nov;86(5):1115-20. doi: 10.1111/cbdd.12579. Epub 2015 May 28. Chem Biol Drug Des. 2015. PMID: 25951978
-
Investigating 1,2,3,4,5,6-hexahydroazepino[4,3-b]indole as scaffold of butyrylcholinesterase-selective inhibitors with additional neuroprotective activities for Alzheimer's disease.Eur J Med Chem. 2019 Sep 1;177:414-424. doi: 10.1016/j.ejmech.2019.05.062. Epub 2019 May 28. Eur J Med Chem. 2019. PMID: 31158754
-
Cholinesterase Inhibitory Activity of Some semi-Rigid Spiro Heterocycles: POM Analyses and Crystalline Structure of Pharmacophore Site.Mini Rev Med Chem. 2018;18(8):711-716. doi: 10.2174/1389557517666170713114039. Mini Rev Med Chem. 2018. PMID: 28714400 Review.
-
A Comprehensive Review of Cholinesterase Modeling and Simulation.Biomolecules. 2021 Apr 15;11(4):580. doi: 10.3390/biom11040580. Biomolecules. 2021. PMID: 33920972 Free PMC article. Review.
Cited by
-
Profiling Novel Quinuclidine-Based Derivatives as Potential Anticholinesterase Drugs: Enzyme Inhibition and Effects on Cell Viability.Int J Mol Sci. 2023 Dec 21;25(1):155. doi: 10.3390/ijms25010155. Int J Mol Sci. 2023. PMID: 38203326 Free PMC article.
References
-
- Knowles R.B., Wyart C., Buldyrev S.V., Cruz L., Urbanc B., Hasselmo M.E., Stanley H.E., Hyman B.T. Plaque-induced neurite abnormalities: Implications for disruption of neural networks in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 1999;96:5274–5279. doi: 10.1073/pnas.96.9.5274. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

