Self-Assembly of Asymmetrically Functionalized Titania Nanoparticles into Nanoshells
- PMID: 33138284
- PMCID: PMC7662802
- DOI: 10.3390/ma13214856
Self-Assembly of Asymmetrically Functionalized Titania Nanoparticles into Nanoshells
Abstract
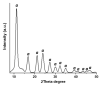
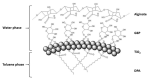
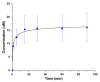
Titania (anatase) nanoparticles were anisotropically functionalized in water-toluene Pickering emulsions to self-assemble into nanoshells with diameters from 500 nm to 3 μm as candidates for encapsulation of drugs and other compounds. The water-phase contained a hydrophilic ligand, glucose-6-phosphate, while the toluene-phase contained a hydrophobic ligand, n-dodecylphosphonic acid. The addition of a dilute sodium alginate suspension that provided electrostatic charge was essential for the self-limited assembly of the nanoshells. The self-assembled spheres were characterized by scanning electron microscopy, elemental mapping, and atomic force microscopy. Drug release studies using tetracycline suggest a rapid release dominated by surface desorption.
Keywords: janus particles; nanoshells (hollow spheres); pickering emulsion; self-assembly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Chen D., Caruso R.A. Recent Progress in the Synthesis of Spherical Titania Nanostructures and Their Applications. Adv. Funct. Mater. 2013;23:1356–1374. doi: 10.1002/adfm.201201880. - DOI
-
- Kotov N.A. Self-Assembly of Inorganic Nanoparticles: Ab ovo(a) Europhys. Lett. 2017;119:66008. doi: 10.1209/0295-5075/119/66008. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

