Crosstalk Pathway between Trehalose Metabolism and Cytokinin Degradation for the Determination of the Number of Berries per Bunch in Grapes
- PMID: 33138306
- PMCID: PMC7693805
- DOI: 10.3390/cells9112378
Crosstalk Pathway between Trehalose Metabolism and Cytokinin Degradation for the Determination of the Number of Berries per Bunch in Grapes
Abstract
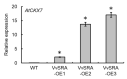
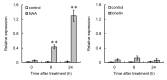
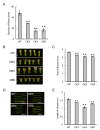
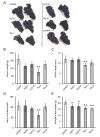
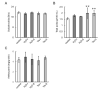
In grapes, the number of flowers per inflorescence determines the compactness of grape bunches. Grape cultivars with tight bunches and thin-skinned berries easily undergo berry splitting, especially in growing areas with heavy rainfall during the grapevine growing season, such as Japan. We report herein that grape cytokinin oxidase/dehydrogenase 5 (VvCKX5) determines the number of berries per inflorescence in grapes. The number of berries per bunch was inversely proportional to the VvCKX5 expression level in juvenile inflorescences among the cultivars tested. VvCKX5 overexpression drastically decreased the number of flower buds per inflorescence in Arabidopsis plants, suggesting that VvCKX5 might be one of the negative regulators of the number of flowers per inflorescence in grapes. Similarly, the overexpression of grape sister of ramose 3 (VvSRA), which encodes trehalose 6-phosphate phosphatase that catalyzes the conversion of trehalose-6-phosphate into trehalose, upregulated AtCKX7 expression in Arabidopsis plants, leading to a decrease in the number of flower buds per Arabidopsis inflorescence. VvCKX5 gene expression was upregulated in grapevine cultured cells and juvenile grape inflorescences treated with trehalose. Finally, injecting trehalose into swelling buds nearing bud break using a microsyringe decreased the number of berries per bunch by half. VvCKX5 overexpression in Arabidopsis plants had no effect on the number of secondary inflorescences from the main inflorescence, and similarly trehalose did not affect pedicel branching on grapevine inflorescences, suggesting that VvCKX5, as well as VvSRA-mediated trehalose metabolism, regulates flower formation but not inflorescence branching. These findings may provide new information on the crosstalk between VvSRA-mediated trehalose metabolism and VvCKX-mediated cytokinin degradation for determining the number of berries per bunch. Furthermore, this study is expected to contribute to the development of innovative cultivation techniques for loosening tight bunches.
Keywords: berry number; cytokinin oxidase/dehydrogenase; grapevine; inflorescence; sister of ramosa3; trehalose.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Changes in transcription of cytokinin metabolism and signalling genes in grape (Vitis vinifera L.) berries are associated with the ripening-related increase in isopentenyladenine.BMC Plant Biol. 2015 Sep 16;15:223. doi: 10.1186/s12870-015-0611-5. BMC Plant Biol. 2015. PMID: 26377914 Free PMC article.
-
Vascular development of the grapevine (Vitis vinifera L.) inflorescence rachis in response to flower number, plant growth regulators and defoliation.J Plant Res. 2017 Sep;130(5):873-883. doi: 10.1007/s10265-017-0944-2. Epub 2017 Apr 18. J Plant Res. 2017. PMID: 28421372
-
Transcriptome analysis of two inflorescence branching mutants reveals cytokinin is an important regulator in controlling inflorescence architecture in the woody plant Jatropha curcas.BMC Plant Biol. 2019 Nov 4;19(1):468. doi: 10.1186/s12870-019-2069-3. BMC Plant Biol. 2019. PMID: 31684864 Free PMC article.
-
A molecular genetic perspective of reproductive development in grapevine.J Exp Bot. 2008;59(10):2579-96. doi: 10.1093/jxb/ern160. J Exp Bot. 2008. PMID: 18596111 Review.
-
Regulation of inflorescence architecture by cytokinins.Front Plant Sci. 2014 Nov 24;5:669. doi: 10.3389/fpls.2014.00669. eCollection 2014. Front Plant Sci. 2014. PMID: 25505480 Free PMC article. Review.
Cited by
-
The lncRNA LOC100257036 and vvimiR156 modulate gibberellin signaling through AGAMOUS during cluster formation in Sistan Yaghooti grape.Sci Rep. 2025 Aug 13;15(1):29687. doi: 10.1038/s41598-025-14984-z. Sci Rep. 2025. PMID: 40804354 Free PMC article.
-
A simple and efficient protocol for transient transformation of sliced grape berries.Protoplasma. 2023 May;260(3):757-766. doi: 10.1007/s00709-022-01810-w. Epub 2022 Sep 12. Protoplasma. 2023. PMID: 36089607
References
-
- IPGRI. UPOV. OIV . Descriptors for Grapevine (Vitis spp.) International Union for the Protection of New Varieties of Plants; Geneva, Switzerland: Office International de la Vigne et du Vin; Paris, France: International Plant Genetic Resources Institute; Rome, Italy: 1997.
-
- Gao X.T., Wu M.H., Sun D., Li H.Q., Chen W.K., Yang H., Liu F.Q., Wang Q.C., Wang Y.Y., Wang J., et al. Effects of gibberellic acid (GA3) application before anthesis on rachis elongation and berry quality and aroma and flavour compounds in Vitis vinifera L. ‘Cabernet Franc’ and ‘Cabernet Sauvignon’ grapes. J. Sci. Food Agric. 2020;100:3729–3740. doi: 10.1002/jsfa.10412. - DOI - PubMed
-
- Miele A., Weaver R.J., Johnson J. Effect of potassium gibberellate on fruit-set and development of Thompson Seedless and Zinfandel grapes. Am. J. Enol. Vitic. 1978;29:79–82.
-
- Nagao A., Sato M. Effects of gibberellic acid spraying on peduncle elongation of Riesling grape. J. ASEV Jpn. 1999;10:12–19.
-
- Tello J., Forneck A. A double-sigmoid model for grapevine bunch compactness development. OENO ONE. 2018;52:307–316. doi: 10.20870/oeno-one.2018.52.4.2132. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

