Defining Coral Bleaching as a Microbial Dysbiosis within the Coral Holobiont
- PMID: 33138319
- PMCID: PMC7692791
- DOI: 10.3390/microorganisms8111682
Defining Coral Bleaching as a Microbial Dysbiosis within the Coral Holobiont
Abstract
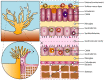
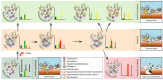
Coral microbiomes are critical to holobiont health and functioning, but the stability of host-microbial interactions is fragile, easily shifting from eubiosis to dysbiosis. The heat-induced breakdown of the symbiosis between the host and its dinoflagellate algae (that is, "bleaching"), is one of the most devastating outcomes for reef ecosystems. Yet, bleaching tolerance has been observed in some coral species. This review provides an overview of the holobiont's diversity, explores coral thermal tolerance in relation to their associated microorganisms, discusses the hypothesis of adaptive dysbiosis as a mechanism of environmental adaptation, mentions potential solutions to mitigate bleaching, and suggests new research avenues. More specifically, we define coral bleaching as the succession of three holobiont stages, where the microbiota can (i) maintain essential functions for holobiont homeostasis during stress and/or (ii) act as a buffer to mitigate bleaching by favoring the recruitment of thermally tolerant Symbiodiniaceae species (adaptive dysbiosis), and where (iii) environmental stressors exceed the buffering capacity of both microbial and dinoflagellate partners leading to coral death.
Keywords: Symbiodiniaceae; adaptive dysbiosis hypothesis; coral bleaching; holobiont; microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The microbiome dynamics and interaction of endosymbiotic Symbiodiniaceae and fungi are associated with thermal bleaching susceptibility of coral holobionts.Appl Environ Microbiol. 2024 Apr 17;90(4):e0193923. doi: 10.1128/aem.01939-23. Epub 2024 Mar 6. Appl Environ Microbiol. 2024. PMID: 38445866 Free PMC article.
-
A Phylogeny-Informed Analysis of the Global Coral-Symbiodiniaceae Interaction Network Reveals that Traits Correlated with Thermal Bleaching Are Specific to Symbiont Transmission Mode.mSystems. 2021 May 4;6(3):e00266-21. doi: 10.1128/mSystems.00266-21. mSystems. 2021. PMID: 33947806 Free PMC article.
-
The trace metal economy of the coral holobiont: supplies, demands and exchanges.Biol Rev Camb Philos Soc. 2023 Apr;98(2):623-642. doi: 10.1111/brv.12922. Epub 2022 Nov 22. Biol Rev Camb Philos Soc. 2023. PMID: 36897260 Review.
-
Consistent responses of coral microbiome to acute and chronic heat stress exposures.Mar Environ Res. 2023 Mar;185:105900. doi: 10.1016/j.marenvres.2023.105900. Epub 2023 Jan 31. Mar Environ Res. 2023. PMID: 36731191
-
Symbiodiniaceae-bacteria interactions: rethinking metabolite exchange in reef-building corals as multi-partner metabolic networks.Environ Microbiol. 2020 May;22(5):1675-1687. doi: 10.1111/1462-2920.14918. Epub 2020 Jan 23. Environ Microbiol. 2020. PMID: 31943674 Review.
Cited by
-
Coral mucus as a reservoir of bacteriophages targeting Vibrio pathogens.ISME J. 2024 Jan 8;18(1):wrae017. doi: 10.1093/ismejo/wrae017. ISME J. 2024. PMID: 38366190 Free PMC article.
-
Marine protected areas do not buffer corals from bleaching under global warming.BMC Ecol Evol. 2022 May 4;22(1):58. doi: 10.1186/s12862-022-02011-y. BMC Ecol Evol. 2022. PMID: 35508975 Free PMC article.
-
Coral probiotics induce tissue-specific and putative beneficial microbiome restructuring in a coral-dwelling fish.ISME Commun. 2025 Mar 22;5(1):ycaf052. doi: 10.1093/ismeco/ycaf052. eCollection 2025 Jan. ISME Commun. 2025. PMID: 40230573 Free PMC article.
-
Symbiodiniaceae photophysiology and stress resilience is enhanced by microbial associations.Sci Rep. 2023 Nov 25;13(1):20724. doi: 10.1038/s41598-023-48020-9. Sci Rep. 2023. PMID: 38007500 Free PMC article.
-
Seasonal dynamics and environmental drivers of tissue and mucus microbiomes in the staghorn coral Acropora pulchra.PeerJ. 2024 May 30;12:e17421. doi: 10.7717/peerj.17421. eCollection 2024. PeerJ. 2024. PMID: 38827308 Free PMC article.
References
-
- Moberg F., Folke C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999;29:215–233. doi: 10.1016/S0921-8009(99)00009-9. - DOI
-
- Spalding M., Spalding M.D., Ravilious C., Green E.P. World Atlas of Coral Reefs. University of California Press; Berkeley, CA, USA: 2001.
-
- Chen P.-Y., Chen C.-C., Chu L., McCarl B. Evaluating the economic damage of climate change on global coral reefs. Glob. Environ. Chang. 2015;30:12–20. doi: 10.1016/j.gloenvcha.2014.10.011. - DOI
-
- Odum H.T., Odum E.P. Trophic structure and productivity of a windward coral reef community on Eniwetok Atoll. Ecol. Monogr. 1955;25:291–320. doi: 10.2307/1943285. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

