Standardization of Microcomputed Tomography for Tracheal Tissue Engineering Analysis
- PMID: 33138726
- PMCID: PMC7698849
- DOI: 10.1089/ten.TEC.2020.0211
Standardization of Microcomputed Tomography for Tracheal Tissue Engineering Analysis
Abstract
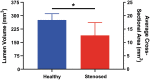
Tracheal tissue engineering has become an active area of interest among clinical and scientific communities; however, methods to evaluate success of in vivo tissue-engineered solutions remain primarily qualitative. These evaluation methods have generally relied on the use of photographs to qualitatively demonstrate tracheal patency, endoscopy to image healing over time, and histology to determine the quality of the regenerated extracellular matrix. Although those generally qualitative methods are valuable, they alone may be insufficient. Therefore, to quantitatively assess tracheal regeneration, we recommend the inclusion of microcomputed tomography (μCT) to quantify tracheal patency as a standard outcome analysis. To establish a standard of practice for quantitative μCT assessment for tracheal tissue engineering, we recommend selecting a constant length to quantify airway volume. Dividing airway volumes by a constant length provides an average cross-sectional area for comparing groups. We caution against selecting a length that is unjustifiably large, which may result in artificially inflating the average cross-sectional area and thereby diminishing the ability to detect actual differences between a test group and a healthy control. Therefore, we recommend selecting a length for μCT assessment that corresponds to the length of the defect region. We further recommend quantifying the minimum cross-sectional area, which does not depend on the length, but has functional implications for breathing. We present empirical data to elucidate the rationale for these recommendations. These empirical data may at first glance appear as expected and unsurprising. However, these standard methods for performing μCT and presentation of results do not yet exist in the literature, and are necessary to improve reporting within the field. Quantitative analyses will better enable comparisons between future publications within the tracheal tissue engineering community and empower a more rigorous assessment of results. Impact statement The current study argues for the standardization of microcomputed tomography (μCT) as a quantitative method for evaluating tracheal tissue-engineered solutions in vivo or ex vivo. The field of tracheal tissue engineering has generally relied on the use of qualitative methods for determining tracheal patency. A standardized quantitative evaluation method currently does not exist. The standardization of μCT for evaluation of in vivo studies would enable a more robust characterization and allow comparisons between groups within the field. The impact of standardized methods within the tracheal tissue engineering field as presented in the current study would greatly improve the quality of published work.
Keywords: microcomputed tomography; trachea; tracheal lumen; tracheal stenosis; tracheal tissue engineering.
Conflict of interest statement
No competing financial interests to disclose.
Figures


References
-
- Law J.X., Liau L.L., Aminuddin B.S., and Ruszymah B.H.. Tissue-engineered trachea: a review. Int J Pediatr Otorhinolaryngol 91, 55, 2016 - PubMed
-
- Ott L.M., Weatherly R.A., and Detamore M.S.. Overview of tracheal tissue engineering: clinical need drives the laboratory approach. Ann Biomed Eng 39, 2091, 2011 - PubMed
-
- Dhasmana A., Singh A., and Rawal S.. Biomedical grafts for tracheal tissue repairing and regeneration “Tracheal tissue engineering: an overview”. J Tissue Eng Regen Med 14, 653, 2020 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

