Enhancing the antibacterial activity of antimicrobial peptide PMAP-37(F34-R) by cholesterol modification
- PMID: 33138816
- PMCID: PMC7607875
- DOI: 10.1186/s12917-020-02630-x
Enhancing the antibacterial activity of antimicrobial peptide PMAP-37(F34-R) by cholesterol modification
Abstract
Background: The problem of increasing resistance against conventional antibiotics has drawn people's attention. Therefore, the development of novel antibacterial agents with effective and safe therapeutic effects is imminent. Antimicrobial peptides (AMPs) are considered a promising class of antibacterial agents due to their broad antibacterial spectrum.
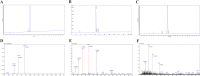
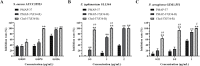
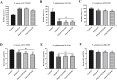
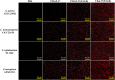
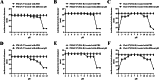
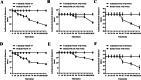
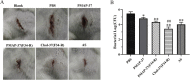
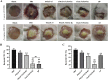
Results: In this study, on the basis of our previously studied peptide PMAP-37(F34-R), a novel antimicrobial peptide Chol-37(F34-R) was developed by N-terminal cholesterol modification to increase hydrophobicity. We observed that the N-terminal cholesterol-modified Chol-37(F34-R) showed higher antimicrobial activity than PMAP-37(F34-R) in vitro. Chol-37(F34-R) also exhibited effective anti-biofilm activity and may kill bacteria by improving the permeability of their membranes. Chol-37(F34-R) exerted high stability in different pH, salt, serum, and boiling water environments. Chol-37(F34-R) also showed no hemolytic activity and substantially low toxicity. Furthermore, Chol-37(F34-R) exhibited good potency of bacteria eradication and promoted wound healing and abscess reduction in infected mice. Meanwhile, in S. aureus ATCC25923-infected peritonitis model, Chol-37(F34-R) exhibited an impressive therapeutic effect by reducing the decrease in systemic bacterial burden and alleviating organ damage.
Conclusions: Our findings suggested that the N-terminal cholesterol modification of PMAP-37(F34-R) could improve antibacterial activity. Chol-37(F34-R) displayed excellent bactericidal efficacy and impressive therapeutic effect in vivo. Thus, Chol-37(F34-R) may be a candidate for antimicrobial agents against microbial infection in the clinic.
Keywords: Antibacterial activity; Antimicrobial peptide PMAP-37(F34-R); Cholesterol; Hydrophobicity; Therapeutic efficacy.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Antimicrobial peptide PMAP-37 analogs: Increasing the positive charge to enhance the antibacterial activity of PMAP-37.J Pept Sci. 2019 Dec;25(12):e3220. doi: 10.1002/psc.3220. J Pept Sci. 2019. PMID: 31858653
-
N-terminal Myristoylation Enhanced the Antimicrobial Activity of Antimicrobial Peptide PMAP-36PW.Front Cell Infect Microbiol. 2020 Aug 27;10:450. doi: 10.3389/fcimb.2020.00450. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32984074 Free PMC article.
-
Antibacterial peptide PMAP-37(F34-R), expressed in Pichia pastoris, is effective against pathogenic bacteria and preserves plums.Microb Cell Fact. 2023 Aug 27;22(1):164. doi: 10.1186/s12934-023-02164-5. Microb Cell Fact. 2023. PMID: 37635252 Free PMC article.
-
Synergism between Host Defence Peptides and Antibiotics Against Bacterial Infections.Curr Top Med Chem. 2020;20(14):1238-1263. doi: 10.2174/1568026620666200303122626. Curr Top Med Chem. 2020. PMID: 32124698 Review.
-
Structure, Function, and Physicochemical Properties of Pore-forming Antimicrobial Peptides.Curr Pharm Biotechnol. 2024;25(8):1041-1057. doi: 10.2174/0113892010194428231017051836. Curr Pharm Biotechnol. 2024. PMID: 37921126 Review.
Cited by
-
A Thermostable, Modified Cathelicidin-Derived Peptide With Enhanced Membrane-Active Activity Against Salmonella enterica serovar Typhimurium.Front Microbiol. 2021 Jan 13;11:592220. doi: 10.3389/fmicb.2020.592220. eCollection 2020. Front Microbiol. 2021. PMID: 33519729 Free PMC article.
-
The Contribution of Antimicrobial Peptides to Immune Cell Function: A Review of Recent Advances.Pharmaceutics. 2023 Sep 4;15(9):2278. doi: 10.3390/pharmaceutics15092278. Pharmaceutics. 2023. PMID: 37765247 Free PMC article. Review.
-
Sustained release of alginate hydrogel containing antimicrobial peptide Chol-37(F34-R) in vitro and its effect on wound healing in murine model of Pseudomonas aeruginosa infection.J Vet Sci. 2023 May;24(3):e44. doi: 10.4142/jvs.22319. J Vet Sci. 2023. PMID: 37271512 Free PMC article.
-
A Comprehensive Review of Recent Research into the Effects of Antimicrobial Peptides on Biofilms-January 2020 to September 2023.Antibiotics (Basel). 2024 Apr 9;13(4):343. doi: 10.3390/antibiotics13040343. Antibiotics (Basel). 2024. PMID: 38667019 Free PMC article. Review.
-
Phytochemical Composition and Antimicrobial Efficacy of Salvadora persica Root Extracts Against Carbapenem-Resistant Acinetobacter baumannii.Cureus. 2024 Apr 20;16(4):e58660. doi: 10.7759/cureus.58660. eCollection 2024 Apr. Cureus. 2024. PMID: 38774172 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

