Health-related quality of life and work productivity in UK patients with HER2-positive breast cancer: a cross-sectional study evaluating the relationships between disease and treatment stage
- PMID: 33138835
- PMCID: PMC7607622
- DOI: 10.1186/s12955-020-01603-w
Health-related quality of life and work productivity in UK patients with HER2-positive breast cancer: a cross-sectional study evaluating the relationships between disease and treatment stage
Abstract
Background: The impact of different disease stages and treatment for human epidermal growth factor 2 positive (HER2-positive) breast cancer (BC) on work productivity and health-related quality of life (HRQoL) is poorly understood.
Methods: This was a UK cross-sectional study of 299 adult patients with HER2-positive early or metastatic BC (NCT03099200). Productivity was assessed using the work productivity and activity impairment scale; HRQoL was measured using EuroQol-5 Dimensions-5 levels (EQ-5D-5L), and Functional Assessment of Cancer Therapy Breast (FACT-G and -B) instruments. Three balanced patient groups were recruited: (1) early BC on treatment post-surgery, (2) early BC after completion of adjuvant treatment, (3) during metastatic BC treatment. Between-group comparisons were performed using an analysis of variance.
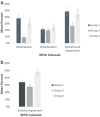
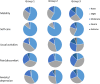
Results: Group 1 comprised 89 patients, Group 2, 108 and Group 3, 102. Age, ethnicity and comorbidities were similar across groups. Patients in Group 3 reported more often being unable to work (significant Bonferroni adjusted p < 0.003). Proportions of employed patients were 50.6%, 50.9% and 27.5% in Groups 1, 2 and 3, respectively. For patients in part-time employment, the number of hours worked was significantly higher in Group 2 patients versus Group 3 (p = 0.002). Group 2 also had significantly lower levels of work absenteeism and overall work impairment compared with Group 1 (p < 0.001). Patients in Group 3 reported worse health utility scores (p ≤ 0.002), moderate or worse problems in the EQ-5D-5L self-care and usual activity domains (p ≤ 0.001), and lower HRQoL as assessed by FACT summary scores (p < 0.001 for FACT-B and -G) than Groups 1 and 2. Poorer HRQoL was significantly associated with higher work impairment (p < 0.001), with the strongest relationships being observed between activity impairment and HRQoL (Pearson's r: 0.67).
Conclusions: Metastatic disease and treatment of HER2-positive BC adversely impacted on work productivity and HRQoL. The results of this study support the idea that being able to delay or prevent the metastatic recurrence of BC, for example by extending the time patients are in remission or at early stage of BC, has wider benefits in terms of patient productivity and HRQoL.
Keywords: Breast cancer; Early disease; Heath-related quality of life; Metastatic disease; Work productivity.
Conflict of interest statement
MV: consulting fees from and ownership interest in Roche Products Ltd. AW: advisory and speaker fees from Roche Products Ltd. JR and ABS: employees of York Health Consortium. CB: was an employee of pH Associates at the time this study was conducted. IT: employee of Genentech (a Roche subsidiary). IL and SND: were employees of Roche Products Ltd at the time this study was conducted. PS: organisational grant (to Queen Mary University of London); spouse has received funding from Roche Products Ltd.
Figures
References
-
- Breast cancer statistics. Cancer Research UK. 2015. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s.... Accessed 12 Apr 2018.
-
- Cleeland CS, Mayer M, Dreyer NA, Yim YM, Yu E, Su Z, et al. Impact of symptom burden on work-related abilities in patients with locally recurrent or metastatic breast cancer: results from a substudy of the VIRGO observational cohort study. Breast. 2014;23:763–769. doi: 10.1016/j.breast.2014.08.004. - DOI - PubMed
-
- Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi: 10.1016/S0140-6736(16)32616-2. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

