Human adipose mesenchymal stem cells modulate myeloid cells toward an anti-inflammatory and reparative phenotype: role of IL-6 and PGE2
- PMID: 33138862
- PMCID: PMC7607855
- DOI: 10.1186/s13287-020-01975-2
Human adipose mesenchymal stem cells modulate myeloid cells toward an anti-inflammatory and reparative phenotype: role of IL-6 and PGE2
Abstract
Background: Mesenchymal stem cells (MSCs) activate the endogenous immune regulatory system, inducing a therapeutic effect in recipients. MSCs have demonstrated the ability to modulate the differentiation of myeloid cells toward a phagocytic and anti-inflammatory profile. Allogeneic, adipose-derived MSCs (ASCs) have been investigated for the management of complex perianal fistula, with darvadstrocel being the first ASC therapy approved in Europe in March 2018. Additionally, ASCs are being explored as a potential treatment in other indications. Yet, despite these clinical advances, their mechanism of action is only partially understood.
Methods: Freshly isolated human monocytes from the peripheral blood were differentiated in vitro toward M0 non-polarized macrophages (Mphs), M1 pro-inflammatory Mphs, M2 anti-inflammatory Mphs, or mature dendritic cells (mDCs) in the presence or absence of ASCs, in non-contact conditions. The phenotype and function of the differentiated myeloid populations were determined by flow cytometry, and their secretome was analyzed by OLINK technology. We also investigated the capacity of ASCs to modulate the phenotype and function of terminally differentiated M1 Mphs. The role of soluble factors interleukin (IL)-6 and prostaglandin E2 (PGE2) on the ability of ASCs to modulate myeloid cells was assessed using neutralization assays, CRISPR/Cas9 knock-down of cyclooxygenase 2 (COX-2), and ASC-conditioned medium assays using pro-inflammatory stimulus.
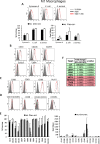
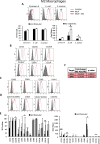
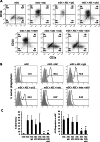
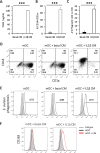
Results: Co-culture of monocytes in the presence of ASCs resulted in the polarization of Mphs and mDCs toward an anti-inflammatory and phagocytic phenotype. This was characterized by an increase in phagocytic receptors on the cell surface of Mphs (M0, M1, and M2) and mDCs, as well as modulation of chemokine receptors and reduced expression of pro-inflammatory, co-stimulatory molecules. ASCs also modulated the secretome of Mphs and mDCs, demonstrated by reduced expression of pro-inflammatory factors and increased expression of anti-inflammatory and reparative factors. Chemical inhibition of PGE2 with indomethacin abolished this modulatory effect, whereas treatment with a neutralizing anti-IL-6 antibody resulted in a partial abolishment. The knock-down of COX-2 in ASCs and the use of IL-1β-activated ASC-conditioned media confirmed the key role of PGE2 in ASC-mediated myeloid modulation. In our in vitro experimental settings, ASCs failed to modulate the phenotype and function of terminally polarized M1 Mphs.
Conclusions: The results demonstrate that ASCs are able to modulate the in vitro differentiation of myeloid cells toward an anti-inflammatory and reparative profile. This modulatory effect was mediated mainly by PGE2 and, to a lesser extent, IL-6.
Keywords: Adipose-derived mesenchymal stem cells; Anti-inflammatory; Dendritic cells; Interleukin 6; Macrophages; Monocytes; Prostaglandin E2.
Conflict of interest statement
All authors except WD are employees of Takeda.
MO-V, PM-C, AA-V, OdlR, and EL are inventors on patents owned by Takeda.
WD is a former employee of TiGenix and is an inventor on patents owned by Takeda.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

