Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer
- PMID: 33138866
- PMCID: PMC7607628
- DOI: 10.1186/s13058-020-01354-y
Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer
Abstract
Background: Treatment options for triple-negative breast cancer remain limited. Activation of the PI3K pathway via loss of PTEN and/or INPP4B is common. Buparlisib is an orally bioavailable, pan-class I PI3K inhibitor. We evaluated the safety and efficacy of buparlisib in patients with metastatic triple-negative breast cancer.
Methods: This was a single-arm phase 2 study enrolling patients with triple-negative metastatic breast cancer. Patients were treated with buparlisib at a starting dose of 100 mg daily. The primary endpoint was clinical benefit, defined as confirmed complete response (CR), partial response (PR), or stable disease (SD) for ≥ 4 months, per RECIST 1.1. Secondary endpoints included progression-free survival (PFS), overall survival (OS), and toxicity. A subset of patients underwent pre- and on-treatment tumor tissue biopsies for correlative studies.
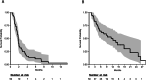
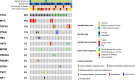
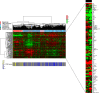
Results: Fifty patients were enrolled. Median number of cycles was 2 (range 1-10). The clinical benefit rate was 12% (6 patients, all SD ≥ 4 months). Median PFS was 1.8 months (95% confidence interval [CI] 1.6-2.3). Median OS was 11.2 months (95% CI 6.2-25). The most frequent adverse events were fatigue (58% all grades, 8% grade 3), nausea (34% all grades, none grade 3), hyperglycemia (34% all grades, 4% grade 3), and anorexia (30% all grades, 2% grade 3). Eighteen percent of patients experienced depression (12% grade 1, 6% grade 2) and anxiety (10% grade 1, 8% grade 2). Alterations in PIK3CA/AKT1/PTEN were present in 6/27 patients with available targeted DNA sequencing (MSK-IMPACT), 3 of whom achieved SD as best overall response though none with clinical benefit ≥ 4 months. Of five patients with paired baseline and on-treatment biopsies, reverse phase protein arrays (RPPA) analysis demonstrated reduction of S6 phosphorylation in 2 of 3 patients who achieved SD, and in none of the patients with progressive disease.
Conclusions: Buparlisib was associated with prolonged SD in a very small subset of patients with triple-negative breast cancer; however, no confirmed objective responses were observed. Downmodulation of key nodes in the PI3K pathway was observed in patients who achieved SD. PI3K pathway inhibition alone may be insufficient as a therapeutic strategy for triple-negative breast cancer.
Trial registration: NCT01790932 . Registered on 13 February 2013; NCT01629615 . Registered on 27 June 2012.
Keywords: BKM120; Buparlisib; PI3K pathway; Phase 1; Triple-negative breast cancer.
Conflict of interest statement
C.S. reports the following personal financial interests: C.S. has served as consultant, participated in advisory boards or received travel grants from AstraZeneca, Celgene, Daiichi Sankyo, Eisai, F. Hoffmann-La Roche Ltd., Genomic Health, Merck, Sharp and Dhome España SA, Novartis, Pfizer, Philips Healthwork, Pierre Fabre, prIME Oncology, Puma, Synthon, and Sanofi Aventis. C.S. reports the following institutional financial interests, paid directly to Institution: AstraZeneca, Daiichi Sankyo, Eli Lilly and Company, Genentech, Immunomedics, Macrogenics, Merck, Sharp and Dhome España S.A., Novartis, Pfizer, Piqur Therapeutics, Puma, Roche, Synthon and Zenith Pharma.
R.B-S. has served as an advisor/consultant to Eli Lilly and Roche and has received honoraria from Eli Lilly, Roche, Bristol-Myers Squib, Novartis, Pfizer, and travel, accommodations, or expenses from Roche.
L.C.C. is a founder and member of the SAB and holds equity in Agios Pharmaceuticals and Petra Pharmaceuticals, companies developing drugs for treating cancer. The laboratory of L.C.C also receives funding from Petra.
D.B.S. has consulted with and received honoraria from Pfizer, Loxo Oncology, Lilly Oncology, Illumina, and Vivideon Therapuetics.
B.B. has participated in advisory boards for MSD, Mylan, Roche, and Novartis.
V.S. has received non-commercial support from Novartis.
C.A. serves or has served as an advisor to Novartis, Lilly, Sanofi, RADIUS, ABBVIE, TAIHO Oncology, PUMA Biotechnology, Merck, H3Biomedicine, Symphogen, OrigiMed, Immunomedics, Petra Pharma, G1 Therapeutics, Athenex, and Daiichi Sankyo. C.A. receives or has received research grant support from Pfizer, Lilly, RADIUS, Bayer, Takeda, PUMA Biotechnology, and Symphogen. C.A. holds stock options in Provista and Y-TRAP and serves in the Scientific Advisory Board of the Komen Foundation.
N.U.L. receives or has received research support paid directly to the Institution from Genentech, Pfizer, Merck, and Seattle Genetics. N.U.L. has served as a consultant or participated in advisory boards for Puma, Seattle Genetics, Daiichi Sankyo, Denali Therapeutics, and California Institute for Regenerative Medicine. N.U.L. has received travel expense reimbursement from Puma and Seattle Genetics.
All other authors declare no relevant conflicts of interest.
Figures

References
-
- Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong YN, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118(22):5463–5472. doi: 10.1002/cncr.27581. - DOI - PMC - PubMed
-
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer. 2007;109(9):1721–1728. doi: 10.1002/cncr.22618. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

