New Perspectives on Antimicrobial Agents: Remdesivir Treatment for COVID-19
- PMID: 33139290
- PMCID: PMC7927874
- DOI: 10.1128/AAC.01814-20
New Perspectives on Antimicrobial Agents: Remdesivir Treatment for COVID-19
Abstract
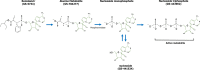
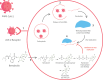
Remdesivir was recently approved by the Food and Drug Administration for the treatment of hospitalized patients with coronavirus disease 2019 (COVID-19). Remdesivir is the prodrug of an adenosine analogue that inhibits viral replication of several RNA virus families, including Coronaviridae Preclinical data in animal models of coronavirus diseases, including COVID-19, have demonstrated that early treatment with remdesivir leads to improved survival, decreased lung injury, and decreased levels of viral RNA. Recent clinical data have demonstrated the clinical activity of remdesivir in terms of faster time to recovery in patients with severe COVID-19 and higher odds of improved clinical status in patients with moderate COVID-19. Here, clinical trials published to date are presented and appraised. Remdesivir's potential benefits and its favorable adverse-event profile make it an option for the treatment of COVID-19. This article examines the available literature describing remdesivir's pharmacology, pharmacokinetics, and preclinical and clinical data.
Keywords: COVID-19; SARS-CoV-2; antiviral; remdesivir.
Copyright © 2020 American Society for Microbiology.
Figures
References
-
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. doi:10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

