ADAP1/Centaurin-α1 Negatively Regulates Dendritic Spine Function and Memory Formation in the Hippocampus
- PMID: 33139322
- PMCID: PMC7808333
- DOI: 10.1523/ENEURO.0111-20.2020
ADAP1/Centaurin-α1 Negatively Regulates Dendritic Spine Function and Memory Formation in the Hippocampus
Abstract
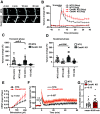
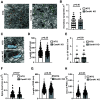
ADAP1/Centaurin-α1 (CentA1) functions as an Arf6 GTPase-activating protein highly enriched in the brain. Previous studies demonstrated the involvement of CentA1 in brain function as a regulator of dendritic differentiation and a potential mediator of Alzheimer's disease (AD) pathogenesis. To better understand the neurobiological functions of CentA1 signaling in the brain, we developed Centa1 knock-out (KO) mice. The KO animals showed neither brain development nor synaptic ultrastructure deficits in the hippocampus. However, they exhibited significantly higher density and enhanced structural plasticity of dendritic spines in the CA1 region of the hippocampus compared with non-transgenic (NTG) littermates. Moreover, the deletion of Centa1 improved performance in the object-in-place (OIP) spatial memory task. These results suggest that CentA1 functions as a negative regulator of spine density and plasticity, and of hippocampus-dependent memory formation. Thus, CentA1 and its downstream signaling may serve as a potential therapeutic target to prevent memory decline associated with aging and brain disorders.
Keywords: ADAP1/Centaurin-α1; Arf6; dendritic spines; hippocampus; learning and memory.
Copyright © 2021 Szatmari et al.
Figures



Similar articles
-
Loss of ADAP1/CentA1 Protects Against Autoimmune Demyelination.FASEB J. 2025 May 15;39(9):e70604. doi: 10.1096/fj.202403078R. FASEB J. 2025. PMID: 40326762 Free PMC article.
-
Centaurin-α1-Ras-Elk-1 signaling at mitochondria mediates β-amyloid-induced synaptic dysfunction.J Neurosci. 2013 Mar 20;33(12):5367-74. doi: 10.1523/JNEUROSCI.2641-12.2013. J Neurosci. 2013. PMID: 23516302 Free PMC article.
-
The neuronal Arf GAP centaurin alpha1 modulates dendritic differentiation.J Cell Sci. 2007 Aug 1;120(Pt 15):2683-93. doi: 10.1242/jcs.006346. Epub 2007 Jul 17. J Cell Sci. 2007. PMID: 17635995 Free PMC article.
-
Functions of the neuron-specific protein ADAP1 (centaurin-α1) in neuronal differentiation and neurodegenerative diseases, with an overview of structural and biochemical properties of ADAP1.Biol Chem. 2014 Nov 1;395(11):1321-40. doi: 10.1515/hsz-2014-0107. Biol Chem. 2014. PMID: 24854535 Review.
-
RanBP9 overexpression accelerates loss of dendritic spines in a mouse model of Alzheimer's disease.Neurobiol Dis. 2014 Sep;69:169-79. doi: 10.1016/j.nbd.2014.05.029. Epub 2014 Jun 2. Neurobiol Dis. 2014. PMID: 24892886 Free PMC article.
Cited by
-
PAK3 downregulation induces cognitive impairment following cranial irradiation.Elife. 2023 Dec 22;12:RP89221. doi: 10.7554/eLife.89221. Elife. 2023. PMID: 38131292 Free PMC article.
-
Human amygdala involvement in Alzheimer's disease revealed by stereological and dia-PASEF analysis.Brain Pathol. 2023 Sep;33(5):e13180. doi: 10.1111/bpa.13180. Epub 2023 Jun 18. Brain Pathol. 2023. PMID: 37331354 Free PMC article.
-
Establishment and characterization of adap1-deficient zebrafish.Dev Growth Differ. 2025 Apr;67(3):165-173. doi: 10.1111/dgd.70004. Epub 2025 Mar 15. Dev Growth Differ. 2025. PMID: 40088134 Free PMC article.
-
Effects of high-intensity interval training on physical and cognitive function in middle-aged male mice.Front Aging. 2025 Aug 14;6:1589730. doi: 10.3389/fragi.2025.1589730. eCollection 2025. Front Aging. 2025. PMID: 40894810 Free PMC article.
-
Loss of ADAP1/CentA1 Protects Against Autoimmune Demyelination.FASEB J. 2025 May 15;39(9):e70604. doi: 10.1096/fj.202403078R. FASEB J. 2025. PMID: 40326762 Free PMC article.
References
-
- Barker GR, Warburton EC (2015) Object-in-place associative recognition memory depends on glutamate receptor neurotransmission within two defined hippocampal-cortical circuits: a critical role for AMPA and NMDA receptors in the hippocampus, perirhinal, and prefrontal cortices. Cereb Cortex 25:472–481. 10.1093/cercor/bht245 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
