Improvement in time to treatment, but not time to diagnosis, in patients with mucopolysaccharidosis type I
- PMID: 33139350
- PMCID: PMC8237187
- DOI: 10.1136/archdischild-2020-319040
Improvement in time to treatment, but not time to diagnosis, in patients with mucopolysaccharidosis type I
Abstract
Objective: Early diagnosis and treatment initiation are important factors for successful treatment of mucopolysaccharidosis type I (MPS I). The purpose of this observational study was to assess whether age at diagnosis and time to first treatment for individuals with MPS I have improved over the last 15 years.
Study design: Data from the MPS I Registry (NCT00144794) for individuals with attenuated or severe disease who initiated therapy with laronidase enzyme replacement therapy (ERT) and/or hematopoietic stem cell transplantation (HSCT) between 1 January 2003 and 31 December 2017 were included.
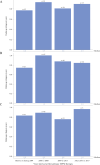
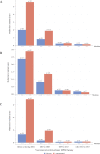
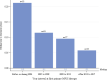
Results: Data were available for 740 individuals with attenuated (n=291) or severe (n=424) MPS I (unknown n=25). Median age at diagnosis for attenuated disease did not change over time and ranged between 4.5 and 6 years of age while the median duration from diagnosis to first ERT decreased from 5.6 years before/during 2004 to 2.4 months in 2014-2017. For severe MPS I treated with HSCT, median age at diagnosis was less than 1 year and median time to first treatment was less than 3 months throughout the 15-year observation period.
Conclusions: Times to diagnosis and HSCT initiation for individuals with severe MPS I were consistent over time. For individuals with attenuated MPS I, the time to ERT initiation after diagnosis has improved substantially in the last 15 years, but median age at diagnosis has not improved. Efforts to improve early diagnosis in attenuated MPS I are needed to ensure that patients receive appropriate treatment at the optimal time.
Keywords: metabolic; monitoring; multidisciplinary team-care; therapeutics.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RG has received consultant/speaker honoraria/fees and/or served on advisory boards for Actelion, Amicus, Biomarin, Inventiva, RegenxBio, Sanofi Genzyme, Sobi, Takeda and Ultragenyx, conducts contracted research for BioMarin, JCR, Lysogene, RegenxBio, Sanofi Genzyme, Takeda, and Ultragenyx and is a member of the International Board of Advisors of the MPS I Registry. NM is a consultant for Biomarin, Sanofi Genzyme, Lysogene, Shire and Sobi, has received grants/research support from Amicus, Biomarin, Sanofi Genzyme and Shire and has received honoraria and/or travel grants from Actelion, Amicus, BioMarin, Chiesi Farmaceutici S.p.A., Sanofi Genzyme and Shire. HK was an employee of Sanofi Genzyme at the time of the study.MD has received consultant/speaker honoraria and/or travel grants from Biomarin, Sanofi Genzyme, Regenxbio, Bluebird Bio, Ultragenyx. JM received consultant/speaker honoraria/fees and served on advisory boards for BioMarin, Denali, Eloxx, RegenxBio, Sangamo, Sanofi Genzyme and Shire, is a PI for a Phase I/II and Phase II/III intrathecal enzyme replacement clinical trials for MPS II and a Phase I/II gene editing clinical trial for MPS II and is a member of the International Board of Advisors of the MPS I Registry.
Figures


Similar articles
-
Early enzyme replacement therapy enables a successful hematopoietic stem cell transplantation in mucopolysaccharidosis type IH: Divergent clinical outcomes in two Japanese siblings.Brain Dev. 2019 Jun;41(6):546-550. doi: 10.1016/j.braindev.2019.01.008. Epub 2019 Feb 10. Brain Dev. 2019. PMID: 30755342
-
Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: results of a European consensus procedure.Orphanet J Rare Dis. 2011 Aug 10;6:55. doi: 10.1186/1750-1172-6-55. Orphanet J Rare Dis. 2011. PMID: 21831279 Free PMC article.
-
Diagnosis and treatment trends in mucopolysaccharidosis I: findings from the MPS I Registry.Eur J Pediatr. 2012 Jun;171(6):911-9. doi: 10.1007/s00431-011-1644-x. Epub 2012 Jan 11. Eur J Pediatr. 2012. PMID: 22234477 Free PMC article.
-
Sleep disordered breathing in mucopolysaccharidosis I: a multivariate analysis of patient, therapeutic and metabolic correlators modifying long term clinical outcome.Orphanet J Rare Dis. 2015 Apr 10;10:42. doi: 10.1186/s13023-015-0255-4. Orphanet J Rare Dis. 2015. PMID: 25887468 Free PMC article.
-
Open issues in Mucopolysaccharidosis type I-Hurler.Orphanet J Rare Dis. 2017 Jun 15;12(1):112. doi: 10.1186/s13023-017-0662-9. Orphanet J Rare Dis. 2017. PMID: 28619065 Free PMC article. Review.
Cited by
-
Light and Shadows in Newborn Screening for Lysosomal Storage Disorders: Eight Years of Experience in Northeast Italy.Int J Neonatal Screen. 2023 Dec 25;10(1):3. doi: 10.3390/ijns10010003. Int J Neonatal Screen. 2023. PMID: 38248631 Free PMC article.
-
Enzyme Replacement Therapy in Mucopolysaccharidosis Type VII: A Three-Year Clinical Outcome Study of the First Taiwanese Case.Diagnostics (Basel). 2025 Feb 14;15(4):464. doi: 10.3390/diagnostics15040464. Diagnostics (Basel). 2025. PMID: 40002615 Free PMC article.
-
A rare partnership: patient community and industry collaboration to shape the impact of real-world evidence on the rare disease ecosystem.Orphanet J Rare Dis. 2024 Jul 10;19(1):262. doi: 10.1186/s13023-024-03262-2. Orphanet J Rare Dis. 2024. PMID: 38987844 Free PMC article. Review.
-
MPSI Manifestations and Treatment Outcome: Skeletal Focus.Int J Mol Sci. 2022 Sep 22;23(19):11168. doi: 10.3390/ijms231911168. Int J Mol Sci. 2022. PMID: 36232472 Free PMC article. Review.
-
Neurological Disease Modeling Using Pluripotent and Multipotent Stem Cells: A Key Step towards Understanding and Treating Mucopolysaccharidoses.Biomedicines. 2023 Apr 21;11(4):1234. doi: 10.3390/biomedicines11041234. Biomedicines. 2023. PMID: 37189853 Free PMC article. Review.
References
-
- Neufeld E, Muenzer J. The mucopolysaccharidoses. In: Schriver C, Beaudet A, Sly W, et al., eds. The metabolic and molecular basis of inherited disease. New York: McGraw Hill, 2001: p. 3421–52.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
