Chlamydia-Specific IgA Secretion in the Female Reproductive Tract Induced via Per-Oral Immunization Confers Protection against Primary Chlamydia Challenge
- PMID: 33139380
- PMCID: PMC7927933
- DOI: 10.1128/IAI.00413-20
Chlamydia-Specific IgA Secretion in the Female Reproductive Tract Induced via Per-Oral Immunization Confers Protection against Primary Chlamydia Challenge
Abstract
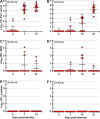
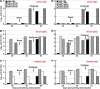
Chlamydia trachomatis is an obligate intracellular pathogen that causes sexually transmitted disease. In women, chlamydial infections may cause pelvic inflammatory disease (PID), ectopic pregnancy, and infertility. The role of antibodies in protection against a primary Chlamydia infection is unclear and was a focus of this work. Using the C. muridarum mouse infection model, we show that intestinal mucosa is infected via intranasal (i.n.) or per-oral (p.o.) Chlamydia inoculation and that unlike the female reproductive tract (FRT) mucosa, it halts systemic Chlamydia dissemination. Moreover, p.o. immunization or infection with Chlamydia confers protection against per-vaginal (p.v.) challenge, resulting in significantly decreased bacterial burden in the FRT, accelerated Chlamydia clearance, and reduced hydrosalpinx pathology. In contrast, subcutaneous (s.c.) immunization conferred no protection against the p.v. challenge. Both p.o. and s.c. immunizations induced Chlamydia-specific serum IgA. However, IgA was found only in the vaginal washes and fecal extracts of p.o.-immunized animals. Following a p.v. challenge, unimmunized control and s.c.-s.c.-immunized animals developed Chlamydia-specific intestinal IgA yet failed to develop IgA in the FRT, indicating that IgA response in the FRT relies on the FRT to gastrointestinal tract (GIT) antigen transport. Vaginal secretions of p.o.-immunized animals neutralize Chlamydia in vivo, resulting in significantly lower Chlamydia burden in the FRT and Chlamydia transport to the GIT. We also show that infection of the GIT is not necessary for induction of protective immunity in the FRT, a finding that is important for the development of p.o. subunit vaccines to target Chlamydia and possibly other sexually transmitted pathogens.
Keywords: Chlamydia; IgA; antibodies; female reproductive tract; mucosa; mucosal vaccines; neutralizing antibodies; vaccination; vaccine.
Copyright © 2020 American Society for Microbiology.
Figures







References
-
- WHO. 2019. Sexually transmitted infections (STIs). https://www.who.int/en/news-room/fact-sheets/detail/sexually-transmitted.... Accessed 16 June 2019.
-
- CDC. 2018. Sexually transmitted disease surveillance 2018. https://www.cdc.gov/std/stats18/default.htm.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

