Using the clinical chemistry score in the emergency department to detect adverse cardiac events: a diagnostic accuracy study
- PMID: 33139388
- PMCID: PMC7608943
- DOI: 10.9778/cmajo.20200047
Using the clinical chemistry score in the emergency department to detect adverse cardiac events: a diagnostic accuracy study
Abstract
Background: The ability to rule out or in a major adverse cardiac event (MACE) in patients with suspected acute coronary syndrome at emergency department (ED) presentation would be beneficial to patient care and the health care system. The clinical chemistry score (CCS) was evaluated in this context.
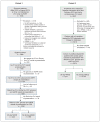
Methods: This diagnostic accuracy study evaluated 2 different ED cohorts with suspected acute coronary syndrome. For patients in cohort 1, who presented to the ED of 3 hospitals in Hamilton, Ontario, between May and August 2013, retrospective measurements were taken using the Ortho Clinical Diagnostics high-sensitivity cardiac troponin I (hs-cTnI) assay; for patients in cohort 2, who presented to the ED of the same 3 hospitals in Hamilton between November 2012 and February 2013, an ED cardiac presentation blood test panel was performed with the Abbott Diagnostics hs-cTnI assay. The sensitivity and specificity of the CCS (cut-offs of ≥ 1 and 5) and hs-cTnI alone (published cut-offs) were compared for MACE (composite of death, myocardial infarction, unstable angina, revascularization) at 30 days for both cohorts and at 90 days for cohort 2.
Results: The incidence of MACE at 30 days was higher in cohort 1 (n = 1058) (19.4%, 95% confidence interval [CI] 16.8%-22.2%) than in cohort 2 (n = 5974) (14.6%, 95% CI 13.6%-15.6%). In cohort 1, a CCS of 1 or above yielded a sensitivity of 99.5% (95% CI 97.3%-99.9%). The sensitivity with an Ortho hs-cTnI cut-off of 1 ng/L or above was 91.2% (95% CI 86.5%-95.7%). The specificity of a CCS of 5 (97.8%, 95% CI 96.5%-98.7%) was higher than when the overall 99th-percentile cut-off for the Ortho hs-cTnI assay (> 11 ng/L; 90.1%, 95% CI 87.9%-92.0%) was used. A similar pattern was observed in cohort 2 at 30 days and persisted at 90 days with the Abbott hs-cTnI assay.
Interpretation: The CCS derived with 2 different hs-cTnI assays and ED populations yielded higher sensitivity and specificity estimates for MACE than hs-cTnI alone. An intervention study is needed to evaluate the impact of the CCS at both the patient and hospital levels.
Trial registration: ClinicalTrials.gov, no. NCT01994577.
Copyright 2020, Joule Inc. or its licensors.
Conflict of interest statement
Competing interests: Peter Kavsak has received grants from Ortho Clinical Diagnostics and Abbott Laboratories for aspects of the present study. Outside this study, Peter Kavsak has received grants from Abbott Laboratories, Beckman Coulter, Ortho Clinical Diagnostics, Roche Diagnostics and Siemens Healthcare Diagnostics; personal fees from Abbott Laboratories, Beckman Coulter, Randox, Roche Diagnostics and Siemens Healthcare Diagnostics; and nonfinancial support from Randox and Siemens Healthcare Diagnostics. McMaster University has filed patents with Peter Kavsak and Andrew Worster listed as inventors in the acute cardiovascular biomarker field. In particular, a patent related to the data in this study has been filed, for a laboratory score for risk stratification for patients with possible cardiac injury. P.J. Devereaux has received grants from Abbott Diagnostics, Boehringer Ingelheim, Philips Healthcare, Roche Diagnostics and Siemens and product support from Philips Healthcare, outside the present study. No other competing interests were declared.
Figures
References
-
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. - PubMed
-
- Pickering JW, Than MP, Cullen L, et al. Rapid rule-out of acute myocardial infarction with a single high-sensitivity cardiac troponin T measurement below the limit of detection: a collaborative meta-analysis. Ann Intern Med. 2017;166:715–24. - PubMed
-
- Thygesen K, Alpert JS, Jaffe AS, et al. ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018) Eur Heart J. 2019;40:237–69. - PubMed
-
- Greenslade J, Cho E, Van Hise C, et al. Evaluating rapid rule-out of acute myocardial infarction using a high-sensitivity cardiac troponin I assay at presentation. Clin Chem. 2018;64:820–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
