Evaluation of a SARS-CoV-2 Surrogate Virus Neutralization Test for Detection of Antibody in Human, Canine, Cat, and Hamster Sera
- PMID: 33139421
- PMCID: PMC8111130
- DOI: 10.1128/JCM.02504-20
Evaluation of a SARS-CoV-2 Surrogate Virus Neutralization Test for Detection of Antibody in Human, Canine, Cat, and Hamster Sera
Abstract
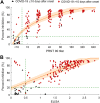
Surrogate neutralization assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that can be done without biosafety level 3 containment and in multiple species are desirable. We evaluate a recently developed surrogate virus neutralization test (sVNT) in comparison to 90% plaque reduction neutralization tests (PRNT90) in human, canine, cat, and hamster sera. With PRNT90 as the reference, sVNT had sensitivity of 98.9% and specificity of 98.8%. Using a panel of immune sera corresponding to other coronaviruses, we confirm the lack of cross-reactivity to other coronaviruses in SARS-CoV-2 sVNT and PRNT90, except for cross-reactivity to SARS-CoV-1 in sVNT.
Keywords: COVID-19; SARS coronavirus 2; SARS-CoV-2; animal; antibody; canine; cat; hamster; human; neutralization; seroepidemiology; serology; surrogate virus neutralization.
Copyright © 2021 American Society for Microbiology.
Figures
References
-
- Newman A, Smith D, Ghai RR, Wallace RM, Torchetti MK, Loiacono C, Murrell LS, Carpenter A, Moroff S, Rooney JA, Behravesh CB. 2020. First reported cases of SARS-CoV-2 infection in companion animals — New York, March–April 2020. MMWR Morb Mortal Wkly Rep 69:710–713. doi:10.15585/mmwr.mm6923e3. - DOI - PMC - PubMed
-
- Oreshkova N, Molenaar RJ, Vreman S, Harders F, Oude Munnink BB, Hakze-van der Honing RW, Gerhards N, Tolsma P, Bouwstra R, Sikkema RS, Tacken MGJ, Rooij MMT, Weesendorp E, Engelsma MY, Bruschke CJM, Smit LAM, Koopmans M, Poel WHM, Stegeman A, Stegeman A. 2020. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Euro Surveill 25:2001005. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.23.... Nadia Oreshkova 1, Robert Jan Molenaar 2, Sandra Vreman 1, Frank Harders 1, Bas B Oude Munnink 3, Renate W Hakze-van der Honing 1, Nora Gerhards 1, Paulien Tolsma 4, Ruth Bouwstra 2, Reina S Sikkema 3, Mirriam Gj Tacken 1, Myrna Mt de Rooij 5, Eefke Weesendorp 1, Marc Y Engelsma 1, Christianne Jm Bruschke 6, Lidwien Am Smit 5, Marion Koopmans 3, Wim Hm van der Poel 1, Arjan Stegeman 7. - DOI - PMC - PubMed
-
- Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, Jiang K, Arunkumar GA, Jurczyszak D, Polanco J, Bermudez-Gonzalez M, Kleiner G, Aydillo T, Miorin L, Fierer DS, Lugo LA, Kojic EM, Stoever J, Liu STH, Cunningham-Rundles C, Felgner PL, Moran T, García-Sastre A, Caplivski D, Cheng AC, Kedzierska K, Vapalahti O, Hepojoki JM, Simon V, Krammer F. 2020. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 26:1033–1036. doi:10.1038/s41591-020-0913-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

