Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19
- PMID: 33139519
- PMCID: PMC7724273
- DOI: 10.1126/scitranslmed.abd3876
Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19
Abstract
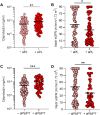
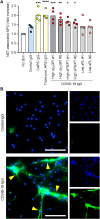
Patients with COVID-19 are at high risk for thrombotic arterial and venous occlusions. Lung histopathology often reveals fibrin-based blockages in the small blood vessels of patients who succumb to the disease. Antiphospholipid syndrome is an acquired and potentially life-threatening thrombophilia in which patients develop pathogenic autoantibodies targeting phospholipids and phospholipid-binding proteins (aPL antibodies). Case series have recently detected aPL antibodies in patients with COVID-19. Here, we measured eight types of aPL antibodies in serum samples from 172 patients hospitalized with COVID-19. These aPL antibodies included anticardiolipin IgG, IgM, and IgA; anti-β2 glycoprotein I IgG, IgM, and IgA; and anti-phosphatidylserine/prothrombin (aPS/PT) IgG and IgM. We detected aPS/PT IgG in 24% of serum samples, anticardiolipin IgM in 23% of samples, and aPS/PT IgM in 18% of samples. Antiphospholipid autoantibodies were present in 52% of serum samples using the manufacturer's threshold and in 30% using a more stringent cutoff (≥40 ELISA-specific units). Higher titers of aPL antibodies were associated with neutrophil hyperactivity, including the release of neutrophil extracellular traps (NETs), higher platelet counts, more severe respiratory disease, and lower clinical estimated glomerular filtration rate. Similar to IgG from patients with antiphospholipid syndrome, IgG fractions isolated from patients with COVID-19 promoted NET release from neutrophils isolated from healthy individuals. Furthermore, injection of IgG purified from COVID-19 patient serum into mice accelerated venous thrombosis in two mouse models. These findings suggest that half of patients hospitalized with COVID-19 become at least transiently positive for aPL antibodies and that these autoantibodies are potentially pathogenic.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures

Update of
-
Prothrombotic antiphospholipid antibodies in COVID-19.medRxiv [Preprint]. 2020 Sep 15:2020.06.15.20131607. doi: 10.1101/2020.06.15.20131607. medRxiv. 2020. Update in: Sci Transl Med. 2020 Nov 18;12(570):eabd3876. doi: 10.1126/scitranslmed.abd3876. PMID: 32587992 Free PMC article. Updated. Preprint.
References
-
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y., Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 180, 934–943 (2020). - PMC - PubMed
-
- Guan W.-j., Ni Z.-y., Hu Y., Liang W.-h., Ou C.-q., He J.-x., Liu L., Shan H., Lei C.-l., Hui D. S. C., Du B., Li L.-j., Zeng G., Yuen K.-Y., Chen R.-c., Tang C.-l., Wang T., Chen P.-y., Xiang J., Li S.-y., Wang J.-l., Liang Z.-j., Peng Y.-x., Wei L., Liu Y., Hu Y.-h., Peng P., Wang J.-m., Liu J.-y., Chen Z., Li G., Zheng Z.-j., Qiu S.-q., Luo J., Ye C.-j., Zhu S.-y., Zhong N.-s.; China Medical Treatment Expert Group for Covid-19 , Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720 (2020). - PMC - PubMed
-
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395, 1054–1062 (2020). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

