LRRC8 family proteins within lysosomes regulate cellular osmoregulation and enhance cell survival to multiple physiological stresses
- PMID: 33139539
- PMCID: PMC7682592
- DOI: 10.1073/pnas.2016539117
LRRC8 family proteins within lysosomes regulate cellular osmoregulation and enhance cell survival to multiple physiological stresses
Abstract
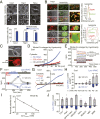
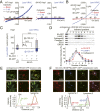
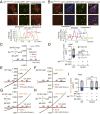
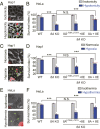
LRRC8 family proteins on the plasma membrane play a critical role in cellular osmoregulation by forming volume-regulated anion channels (VRACs) necessary to prevent necrotic cell death. We demonstrate that intracellular LRRC8 proteins acting within lysosomes also play an essential role in cellular osmoregulation. LRRC8 proteins on lysosome membranes generate large lysosomal volume-regulated anion channel (Lyso-VRAC) currents in response to low cytoplasmic ionic strength conditions. When a double-leucine L706L707 motif at the C terminus of LRRC8A was mutated to alanines, normal plasma membrane VRAC currents were still observed, but Lyso-VRAC currents were absent. We used this targeting mutant, as well as pharmacological tools, to demonstrate that Lyso-VRAC currents are necessary for the formation of large lysosome-derived vacuoles, which store and then expel excess water to maintain cytosolic water homeostasis. Thus, Lyso-VRACs allow lysosomes of mammalian cells to act as the cell`s "bladder." When Lyso-VRAC current was selectively eliminated, the extent of necrotic cell death to sustained stress was greatly increased, not only in response to hypoosmotic stress, but also to hypoxic and hypothermic stresses. Thus Lyso-VRACs play an essential role in enabling cells to mount successful homeostatic responses to multiple stressors.
Keywords: chloride channel; exocytosis; lysosome; osmoregulation; vacuolation.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Intracellular and extracellular loops of LRRC8 are essential for volume-regulated anion channel function.J Gen Physiol. 2018 Jul 2;150(7):1003-1015. doi: 10.1085/jgp.201812016. Epub 2018 May 31. J Gen Physiol. 2018. PMID: 29853476 Free PMC article.
-
Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC.Science. 2014 May 9;344(6184):634-8. doi: 10.1126/science.1252826. Epub 2014 Apr 10. Science. 2014. PMID: 24790029
-
Molecular composition and heterogeneity of the LRRC8-containing swelling-activated osmolyte channels in primary rat astrocytes.J Physiol. 2017 Nov 15;595(22):6939-6951. doi: 10.1113/JP275053. Epub 2017 Sep 12. J Physiol. 2017. PMID: 28833202 Free PMC article.
-
Mechanisms of Activation of LRRC8 Volume Regulated Anion Channels.Cell Physiol Biochem. 2021 Feb 13;55(S1):41-56. doi: 10.33594/000000329. Cell Physiol Biochem. 2021. PMID: 33577730 Review.
-
The volume-regulated anion channel is formed by LRRC8 heteromers – molecular identification and roles in membrane transport and physiology.Biol Chem. 2015 Sep;396(9-10):975-90. doi: 10.1515/hsz-2015-0127. Biol Chem. 2015. PMID: 25868000 Review.
Cited by
-
Impaired Autophagic Clearance with a Gain-of-Function Variant of the Lysosomal Cl-/H+ Exchanger ClC-7.Biomolecules. 2023 Dec 15;13(12):1799. doi: 10.3390/biom13121799. Biomolecules. 2023. PMID: 38136669 Free PMC article.
-
Mitigating Cold Ischemic Injury: HTK, UW and IGL-2 Solution's Role in Enhancing Antioxidant Defence and Reducing Inflammation in Steatotic Livers.Int J Mol Sci. 2024 Aug 28;25(17):9318. doi: 10.3390/ijms25179318. Int J Mol Sci. 2024. PMID: 39273266 Free PMC article.
-
ADP-Ribosylation Post-Translational Modification: An Overview with a Focus on RNA Biology and New Pharmacological Perspectives.Biomolecules. 2022 Mar 13;12(3):443. doi: 10.3390/biom12030443. Biomolecules. 2022. PMID: 35327636 Free PMC article. Review.
-
Electrophysiological Techniques on the Study of Endolysosomal Ion Channels.Handb Exp Pharmacol. 2023;278:217-233. doi: 10.1007/164_2023_638. Handb Exp Pharmacol. 2023. PMID: 36871125
-
Parkinson's disease-risk protein TMEM175 is a proton-activated proton channel in lysosomes.Cell. 2022 Jun 23;185(13):2292-2308.e20. doi: 10.1016/j.cell.2022.05.021. Cell. 2022. PMID: 35750034 Free PMC article.
References
-
- Hoffmann E. K., Lambert I. H., Pedersen S. F., Physiology of cell volume regulation in vertebrates. Physiol. Rev. 89, 193–277 (2009). - PubMed
-
- Bourque C. W., Central mechanisms of osmosensation and systemic osmoregulation. Nat. Rev. Neurosci. 9, 519–531 (2008). - PubMed
-
- Kültz D., Cellular osmoregulation: Beyond ion transport and cell volume. Zoology (Jena) 104, 198–208 (2001). - PubMed
-
- Jentsch T. J., VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat. Rev. Mol. Cell Biol. 17, 293–307 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

