The inhibition of LSD1 via sequestration contributes to tau-mediated neurodegeneration
- PMID: 33139560
- PMCID: PMC7682552
- DOI: 10.1073/pnas.2013552117
The inhibition of LSD1 via sequestration contributes to tau-mediated neurodegeneration
Abstract
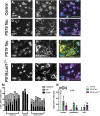
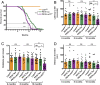
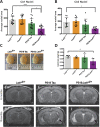
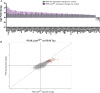
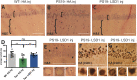
Tauopathies are a class of neurodegenerative diseases associated with pathological tau. Despite many advances in our understanding of these diseases, the direct mechanism through which tau contributes to neurodegeneration remains poorly understood. Previously, our laboratory implicated the histone demethylase LSD1 in tau-induced neurodegeneration by showing that LSD1 localizes to pathological tau aggregates in Alzheimer's disease cases, and that it is continuously required for the survival of hippocampal and cortical neurons in mice. Here, we utilize the P301S tauopathy mouse model to demonstrate that pathological tau can exclude LSD1 from the nucleus in neurons. In addition, we show that reducing LSD1 in these mice is sufficient to highly exacerbate tau-mediated neurodegeneration and tau-induced gene expression changes. Finally, we find that overexpressing LSD1 in the hippocampus of tauopathy mice, even after pathology has formed, is sufficient to significantly delay neurodegeneration and counteract tau-induced expression changes. These results suggest that inhibiting LSD1 via sequestration contributes to tau-mediated neurodegeneration. Thus, LSD1 is a promising therapeutic target for tauopathies such as Alzheimer's disease.
Keywords: Alzheimer’s disease; LSD1; epigenetics; neurodegeneration; tauopathy.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
LSD1 protects against hippocampal and cortical neurodegeneration.Nat Commun. 2017 Oct 9;8(1):805. doi: 10.1038/s41467-017-00922-9. Nat Commun. 2017. PMID: 28993646 Free PMC article.
-
The mTOR Inhibitor Rapamycin Mitigates Perforant Pathway Neurodegeneration and Synapse Loss in a Mouse Model of Early-Stage Alzheimer-Type Tauopathy.PLoS One. 2015 Nov 5;10(11):e0142340. doi: 10.1371/journal.pone.0142340. eCollection 2015. PLoS One. 2015. PMID: 26540269 Free PMC article.
-
Amphiphysin-1 protein level changes associated with tau-mediated neurodegeneration.Neuroreport. 2012 Nov 14;23(16):942-6. doi: 10.1097/WNR.0b013e32835982ce. Neuroreport. 2012. PMID: 22975846 Free PMC article.
-
Tau Seeding Mouse Models with Patient Brain-Derived Aggregates.Int J Mol Sci. 2021 Jun 7;22(11):6132. doi: 10.3390/ijms22116132. Int J Mol Sci. 2021. PMID: 34200180 Free PMC article. Review.
-
Tauopathies and tau oligomers.J Alzheimers Dis. 2013;37(3):565-8. doi: 10.3233/JAD-130653. J Alzheimers Dis. 2013. PMID: 23948895 Review.
Cited by
-
Kdm1a safeguards the topological boundaries of PRC2-repressed genes and prevents aging-related euchromatinization in neurons.Nat Commun. 2024 Mar 7;15(1):1781. doi: 10.1038/s41467-024-45773-3. Nat Commun. 2024. PMID: 38453932 Free PMC article.
-
Co-Aggregation of TDP-43 with Other Pathogenic Proteins and Their Co-Pathologies in Neurodegenerative Diseases.Int J Mol Sci. 2024 Nov 18;25(22):12380. doi: 10.3390/ijms252212380. Int J Mol Sci. 2024. PMID: 39596445 Free PMC article. Review.
-
Demethylase-independent roles of LSD1 in regulating enhancers and cell fate transition.Nat Commun. 2023 Aug 22;14(1):4944. doi: 10.1038/s41467-023-40606-1. Nat Commun. 2023. PMID: 37607921 Free PMC article.
-
A putative role for lncRNAs in epigenetic regulation of memory.Neurochem Int. 2021 Nov;150:105184. doi: 10.1016/j.neuint.2021.105184. Epub 2021 Sep 14. Neurochem Int. 2021. PMID: 34530054 Free PMC article. Review.
-
Role of NAD+ and FAD in Ischemic Stroke Pathophysiology: An Epigenetic Nexus and Expanding Therapeutic Repertoire.Cell Mol Neurobiol. 2023 Jul;43(5):1719-1768. doi: 10.1007/s10571-022-01287-4. Epub 2022 Sep 30. Cell Mol Neurobiol. 2023. PMID: 36180651 Free PMC article. Review.
References
-
- Wang Y., Mandelkow E., Tau in physiology and pathology. Nat. Rev. Neurosci. 17, 5–21 (2016). - PubMed
-
- Holtzman D. M., et al. , Tau: From research to clinical development. Alzheimers Dement. 12, 1033–1039 (2016). - PubMed
-
- Goedert M., Wischik C. M., Crowther R. A., Walker J. E., Klug A., Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of alzheimer disease: Identification as the microtubule-associated protein tau. Proc. Natl. Acad. Sci. U.S.A. 85, 4051–4055 (1988). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

