An evolutionarily acquired microRNA shapes development of mammalian cortical projections
- PMID: 33139574
- PMCID: PMC7682328
- DOI: 10.1073/pnas.2006700117
An evolutionarily acquired microRNA shapes development of mammalian cortical projections
Abstract
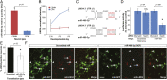
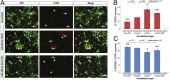
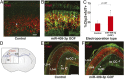
The corticospinal tract is unique to mammals and the corpus callosum is unique to placental mammals (eutherians). The emergence of these structures is thought to underpin the evolutionary acquisition of complex motor and cognitive skills. Corticospinal motor neurons (CSMN) and callosal projection neurons (CPN) are the archetypal projection neurons of the corticospinal tract and corpus callosum, respectively. Although a number of conserved transcriptional regulators of CSMN and CPN development have been identified in vertebrates, none are unique to mammals and most are coexpressed across multiple projection neuron subtypes. Here, we discover 17 CSMN-enriched microRNAs (miRNAs), 15 of which map to a single genomic cluster that is exclusive to eutherians. One of these, miR-409-3p, promotes CSMN subtype identity in part via repression of LMO4, a key transcriptional regulator of CPN development. In vivo, miR-409-3p is sufficient to convert deep-layer CPN into CSMN. This is a demonstration of an evolutionarily acquired miRNA in eutherians that refines cortical projection neuron subtype development. Our findings implicate miRNAs in the eutherians' increase in neuronal subtype and projection diversity, the anatomic underpinnings of their complex behavior.
Keywords: cerebral cortex; cortical development; microRNA; motor neuron; projection neuron.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: C.J.S. and authors J.L.D., V.B.S., V.L., N.G.-N., M.B.W., R.N., Z.H., P.S., T.D.P., and S.T. are affiliated with Stanford University.
Figures


References
-
- Beck P. D., Pospichal M. W., Kaas J. H., Topography, architecture, and connections of somatosensory cortex in opossums: Evidence for five somatosensory areas. J. Comp. Neurol. 366, 109–133 (1996). - PubMed
-
- Frost S. B., Milliken G. W., Plautz E. J., Masterton R. B., Nudo R. J., Somatosensory and motor representations in cerebral cortex of a primitive mammal (Monodelphis domestica): A window into the early evolution of sensorimotor cortex. J. Comp. Neurol. 421, 29–51 (2000). - PubMed
-
- Kaas J. H., Evolution of somatosensory and motor cortex in primates. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 281, 1148–1156 (2004). - PubMed
-
- Mihrshahi R., The corpus callosum as an evolutionary innovation. J. Exp. Zoolog. B Mol. Dev. Evol. 306, 8–17 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS049553/NS/NINDS NIH HHS/United States
- R37 NS041590/NS/NINDS NIH HHS/United States
- R01 NS041590/NS/NINDS NIH HHS/United States
- T32 MH020016/MH/NIMH NIH HHS/United States
- K08 NS091531/NS/NINDS NIH HHS/United States
- R01 NS045523/NS/NINDS NIH HHS/United States
- R01 MH108660/MH/NIMH NIH HHS/United States
- R01 MH108659/MH/NIMH NIH HHS/United States
- R01 NS104055/NS/NINDS NIH HHS/United States
- P30 NS069375/NS/NINDS NIH HHS/United States
- T32 OD011121/OD/NIH HHS/United States
- R21 NS096447/NS/NINDS NIH HHS/United States
- R01 AI069000/AI/NIAID NIH HHS/United States
- R01 NS075672/NS/NINDS NIH HHS/United States
- DP1 NS106665/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

