Anti-S1 MERS-COV IgY Specific Antibodies Decreases Lung Inflammation and Viral Antigen Positive Cells in the Human Transgenic Mouse Model
- PMID: 33139631
- PMCID: PMC7712919
- DOI: 10.3390/vaccines8040634
Anti-S1 MERS-COV IgY Specific Antibodies Decreases Lung Inflammation and Viral Antigen Positive Cells in the Human Transgenic Mouse Model
Abstract
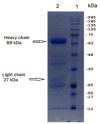
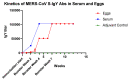
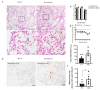
The Middle East respiratory syndrome coronavirus (MERS-CoV) was identified in 2012 and causes severe and often fatal acute respiratory illness in humans. No approved prophylactic and therapeutic interventions are currently available. In this study, we have developed egg yolk antibodies (immunoglobulin Y (IgY)) specific for MERS-CoV spike protein (S1) in order to evaluate their neutralizing efficiency against MERS-CoV infection. S1-specific immunoglobulins were produced by injecting chickens with purified recombinant S1 protein of MERS-CoV at a high titer (5.7 mg/mL egg yolk) at week 7 post immunization. Western blotting and immune-dot blot assays demonstrated that the IgY antibody specifically bound to the MERS-CoV S1 protein. Anti-S1 antibodies were also able to recognize MERS-COV inside cells, as demonstrated by an immunofluorescence assay. Plaque reduction and microneutralization assays showed the neutralization of MERS-COV in Vero cells by anti-S1 IgY antibodies and non-significantly reduced virus titers in the lungs of MERS-CoV-infected mice during early infection, with a nonsignificant decrease in weight loss. However, a statistically significant (p = 0.0196) quantitative reduction in viral antigen expression and marked reduction in inflammation were observed in lung tissue. Collectively, our data suggest that the anti-MERS-CoV S1 IgY could serve as a potential candidate for the passive treatment of MERS-CoV infection.
Keywords: IgY against MERS-COV; MERS-COV; controlling of MERS-COV infection; egg yolk antibodies; passive immunotherapy; vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures





References
-
- Widjaja I., Wang C., van Haperen R., Gutierrez-Alvarez J., van Dieren B., Okba N.M.A., Raj V.S., Li W., Fernandez-Delgado R., Grosveld F., et al. Towards a solution to MERS: Protective human monoclonal antibodies targeting different domains and functions of the MERS-coronavirus spike glycoprotein. Emerg. Microbes Infect. 2019;8:516–530. doi: 10.1080/22221751.2019.1597644. - DOI - PMC - PubMed
-
- World Health Organization Mers Situation Update. [(accessed on 30 October 2020)]; Available online: http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

