Targeting BCL-2 in B-cell malignancies and overcoming therapeutic resistance
- PMID: 33139702
- PMCID: PMC7608616
- DOI: 10.1038/s41419-020-03144-y
Targeting BCL-2 in B-cell malignancies and overcoming therapeutic resistance
Abstract
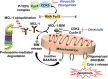
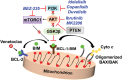
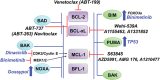
Defects in apoptosis can promote tumorigenesis and impair responses of malignant B cells to chemotherapeutics. Members of the B-cell leukemia/lymphoma-2 (BCL-2) family of proteins are key regulators of the intrinsic, mitochondrial apoptotic pathway. Overexpression of antiapoptotic BCL-2 family proteins is associated with treatment resistance and poor prognosis. Thus, inhibition of BCL-2 family proteins is a rational therapeutic option for malignancies that are dependent on antiapoptotic BCL-2 family proteins. Venetoclax (ABT-199, GDC-0199) is a highly selective BCL-2 inhibitor that represents the first approved agent of this class and is currently widely used in the treatment of chronic lymphocytic leukemia (CLL) as well as acute myeloid leukemia (AML). Despite impressive clinical activity, venetoclax monotherapy for a prolonged duration can lead to drug resistance or loss of dependence on the targeted protein. In this review, we provide an overview of the mechanism of action of BCL-2 inhibition and the role of this approach in the current treatment paradigm of B-cell malignancies. We summarize the drivers of de novo and acquired resistance to venetoclax that are closely associated with complex clonal shifts, interplay of expression and interactions of BCL-2 family members, transcriptional regulators, and metabolic modulators. We also examine how tumors initially resistant to venetoclax become responsive to it following prior therapies. Here, we summarize preclinical data providing a rationale for efficacious combination strategies of venetoclax to overcome therapeutic resistance by a targeted approach directed against alternative antiapoptotic BCL-2 family proteins (MCL-1, BCL-xL), compensatory prosurvival pathways, epigenetic modifiers, and dysregulated cellular metabolism/energetics for durable clinical remissions.
Conflict of interest statement
B.T.H. receives research funding and consulting fees from Abbvie and Genentech. E.D.H. receives funding from Abbvie.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

