Allomorphy as a mechanism of post-translational control of enzyme activity
- PMID: 33139716
- PMCID: PMC7608592
- DOI: 10.1038/s41467-020-19215-9
Allomorphy as a mechanism of post-translational control of enzyme activity
Abstract
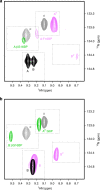
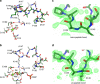
Enzyme regulation is vital for metabolic adaptability in living systems. Fine control of enzyme activity is often delivered through post-translational mechanisms, such as allostery or allokairy. β-phosphoglucomutase (βPGM) from Lactococcus lactis is a phosphoryl transfer enzyme required for complete catabolism of trehalose and maltose, through the isomerisation of β-glucose 1-phosphate to glucose 6-phosphate via β-glucose 1,6-bisphosphate. Surprisingly for a gatekeeper of glycolysis, no fine control mechanism of βPGM has yet been reported. Herein, we describe allomorphy, a post-translational control mechanism of enzyme activity. In βPGM, isomerisation of the K145-P146 peptide bond results in the population of two conformers that have different activities owing to repositioning of the K145 sidechain. In vivo phosphorylating agents, such as fructose 1,6-bisphosphate, generate phosphorylated forms of both conformers, leading to a lag phase in activity until the more active phosphorylated conformer dominates. In contrast, the reaction intermediate β-glucose 1,6-bisphosphate, whose concentration depends on the β-glucose 1-phosphate concentration, couples the conformational switch and the phosphorylation step, resulting in the rapid generation of the more active phosphorylated conformer. In enabling different behaviours for different allomorphic activators, allomorphy allows an organism to maximise its responsiveness to environmental changes while minimising the diversion of valuable metabolites.
Conflict of interest statement
The authors declare no competing interest.
Figures
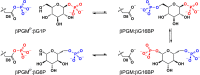
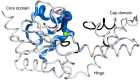


References
Publication types
MeSH terms
Substances
Grants and funding
- BB/P007066/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/S007965/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- X/009906-20-26/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/R000727/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M021637/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources

