Preclinical studies using cisplatin/carboplatin to restore the Enzalutamide sensitivity via degrading the androgen receptor splicing variant 7 (ARv7) to further suppress Enzalutamide resistant prostate cancer
- PMID: 33139720
- PMCID: PMC7606511
- DOI: 10.1038/s41419-020-02970-4
Preclinical studies using cisplatin/carboplatin to restore the Enzalutamide sensitivity via degrading the androgen receptor splicing variant 7 (ARv7) to further suppress Enzalutamide resistant prostate cancer
Abstract
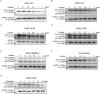
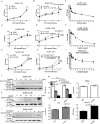
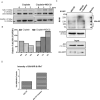
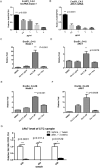
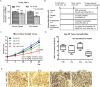
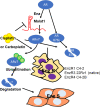
The FDA-approved anti-androgen Enzalutamide (Enz) has been used successfully as the last line therapy to extend castration-resistant prostate cancer (CRPC) patients' survival by an extra 4.8 months. However, CRPC patients eventually develop Enz-resistance that may involve the induction of the androgen receptor (AR) splicing variant ARv7. Here we found that Cisplatin (Cis) or Carboplatin, currently used in chemotherapy/radiation therapy to suppress tumor progression, could restore the Enz sensitivity in multiple Enz-resistant (EnzR) CRPC cells via directly degrading/suppressing the ARv7. Combining Cis or Carboplatin with Enz therapy can also delay the development of Enz-resistance in CRPC C4-2 cells. Mechanism dissection found that Cis or Carboplatin might decrease the ARv7 expression via multiple mechanisms including targeting the lncRNA-Malat1/SF2 RNA splicing complex and increasing ARv7 degradation via altering ubiquitination. Preclinical studies using in vivo mouse model with implanted EnzR1-C4-2 cells also demonstrated that Cis plus Enz therapy resulted in better suppression of EnzR CRPC progression than Enz treatment alone. These results not only unveil the previously unrecognized Cis mechanism to degrade ARv7 via targeting the Malat1/SF2 complex and ubiquitination signals, it may also provide a novel and ready therapy to further suppress the EnzR CRPC progression in the near future.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

