CFAP45 deficiency causes situs abnormalities and asthenospermia by disrupting an axonemal adenine nucleotide homeostasis module
- PMID: 33139725
- PMCID: PMC7606486
- DOI: 10.1038/s41467-020-19113-0
CFAP45 deficiency causes situs abnormalities and asthenospermia by disrupting an axonemal adenine nucleotide homeostasis module
Abstract
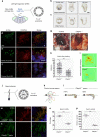
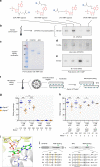
Axonemal dynein ATPases direct ciliary and flagellar beating via adenosine triphosphate (ATP) hydrolysis. The modulatory effect of adenosine monophosphate (AMP) and adenosine diphosphate (ADP) on flagellar beating is not fully understood. Here, we describe a deficiency of cilia and flagella associated protein 45 (CFAP45) in humans and mice that presents a motile ciliopathy featuring situs inversus totalis and asthenospermia. CFAP45-deficient cilia and flagella show normal morphology and axonemal ultrastructure. Proteomic profiling links CFAP45 to an axonemal module including dynein ATPases and adenylate kinase as well as CFAP52, whose mutations cause a similar ciliopathy. CFAP45 binds AMP in vitro, consistent with structural modelling that identifies an AMP-binding interface between CFAP45 and AK8. Microtubule sliding of dyskinetic sperm from Cfap45-/- mice is rescued with the addition of either AMP or ADP with ATP, compared to ATP alone. We propose that CFAP45 supports mammalian ciliary and flagellar beating via an adenine nucleotide homeostasis module.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
The cilia and flagella associated protein CFAP52 orchestrated with CFAP45 is required for sperm motility in mice.J Biol Chem. 2023 Jul;299(7):104858. doi: 10.1016/j.jbc.2023.104858. Epub 2023 May 24. J Biol Chem. 2023. PMID: 37236356 Free PMC article.
-
Defects in the cytoplasmic assembly of axonemal dynein arms cause morphological abnormalities and dysmotility in sperm cells leading to male infertility.PLoS Genet. 2021 Feb 26;17(2):e1009306. doi: 10.1371/journal.pgen.1009306. eCollection 2021 Feb. PLoS Genet. 2021. PMID: 33635866 Free PMC article.
-
Calaxin is required for cilia-driven determination of vertebrate laterality.Commun Biol. 2019 Jun 20;2:226. doi: 10.1038/s42003-019-0462-y. eCollection 2019. Commun Biol. 2019. PMID: 31240264 Free PMC article.
-
Ultrastructure of cilia and flagella - back to the future!Biol Cell. 2011 Jun;103(6):249-70. doi: 10.1042/BC20100139. Biol Cell. 2011. PMID: 21728999 Review.
-
Flagellar and ciliary beating: the proven and the possible.J Cell Sci. 2010 Feb 15;123(Pt 4):519-28. doi: 10.1242/jcs.051326. J Cell Sci. 2010. PMID: 20145000 Review.
Cited by
-
Identification of candidate sex-specific genomic regions in male and female Asian arowana genomes.Gigascience. 2022 Sep 15;11:giac085. doi: 10.1093/gigascience/giac085. Gigascience. 2022. PMID: 36106701 Free PMC article.
-
MR1 overexpression correlates with poor clinical prognosis in glioma patients.Neurooncol Adv. 2021 Feb 20;3(1):vdab034. doi: 10.1093/noajnl/vdab034. eCollection 2021 Jan-Dec. Neurooncol Adv. 2021. PMID: 33948562 Free PMC article.
-
A novel homozygous splicing mutation in AK7 causes multiple morphological abnormalities of sperm flagella in patients from consanguineous Pakistani families.Asian J Androl. 2025 Mar 1;27(2):189-195. doi: 10.4103/aja202471. Epub 2024 Sep 10. Asian J Androl. 2025. PMID: 39254435 Free PMC article.
-
Conserved genes regulating human sex differentiation, gametogenesis and fertilization.J Transl Med. 2024 May 19;22(1):473. doi: 10.1186/s12967-024-05162-2. J Transl Med. 2024. PMID: 38764035 Free PMC article. Review.
-
The inner junction protein CFAP20 functions in motile and non-motile cilia and is critical for vision.Nat Commun. 2022 Nov 3;13(1):6595. doi: 10.1038/s41467-022-33820-w. Nat Commun. 2022. PMID: 36329026 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

