Identification of HMGA2 inhibitors by AlphaScreen-based ultra-high-throughput screening assays
- PMID: 33139812
- PMCID: PMC7606612
- DOI: 10.1038/s41598-020-75890-0
Identification of HMGA2 inhibitors by AlphaScreen-based ultra-high-throughput screening assays
Abstract
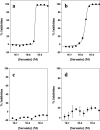
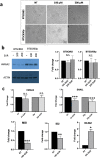
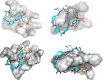
The mammalian high mobility group protein AT-hook 2 (HMGA2) is a multi-functional DNA-binding protein that plays important roles in tumorigenesis and adipogenesis. Previous results showed that HMGA2 is a potential therapeutic target of anticancer and anti-obesity drugs by inhibiting its DNA-binding activities. Here we report the development of a miniaturized, automated AlphaScreen ultra-high-throughput screening assay to identify inhibitors targeting HMGA2-DNA interactions. After screening the LOPAC1280 compound library, we identified several compounds that strongly inhibit HMGA2-DNA interactions including suramin, a century-old, negatively charged antiparasitic drug. Our results show that the inhibition is likely through suramin binding to the "AT-hook" DNA-binding motifs and therefore preventing HMGA2 from binding to the minor groove of AT-rich DNA sequences. Since HMGA1 proteins also carry multiple "AT-hook" DNA-binding motifs, suramin is expected to inhibit HMGA1-DNA interactions as well. Biochemical and biophysical studies show that charge-charge interactions and hydrogen bonding between the suramin sulfonated groups and Arg/Lys residues play critical roles in the binding of suramin to the "AT-hook" DNA-binding motifs. Furthermore, our results suggest that HMGA2 may be one of suramin's cellular targets.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Inhibition of HMGA2 binding to AT-rich DNA by its negatively charged C-terminus.Nucleic Acids Res. 2025 Jan 24;53(3):gkaf035. doi: 10.1093/nar/gkaf035. Nucleic Acids Res. 2025. PMID: 39873271 Free PMC article.
-
The Mammalian High Mobility Group Protein AT-Hook 2 (HMGA2): Biochemical and Biophysical Properties, and Its Association with Adipogenesis.Int J Mol Sci. 2020 May 25;21(10):3710. doi: 10.3390/ijms21103710. Int J Mol Sci. 2020. PMID: 32466162 Free PMC article. Review.
-
A rapid and sensitive high-throughput screening method to identify compounds targeting protein-nucleic acids interactions.Nucleic Acids Res. 2015 Apr 30;43(8):e52. doi: 10.1093/nar/gkv069. Epub 2015 Feb 4. Nucleic Acids Res. 2015. PMID: 25653160 Free PMC article.
-
Specific recognition of AT-rich DNA sequences by the mammalian high mobility group protein AT-hook 2: a SELEX study.Biochemistry. 2007 Nov 13;46(45):13059-66. doi: 10.1021/bi701269s. Epub 2007 Oct 23. Biochemistry. 2007. PMID: 17956125
-
HMGA molecules in neuroblastic tumors.Ann N Y Acad Sci. 2004 Dec;1028:122-32. doi: 10.1196/annals.1322.013. Ann N Y Acad Sci. 2004. PMID: 15650238 Review.
Cited by
-
Aberrant stem cell and developmental programs in pediatric leukemia.Front Cell Dev Biol. 2024 Mar 27;12:1372899. doi: 10.3389/fcell.2024.1372899. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38601080 Free PMC article. Review.
-
Design, synthesis and evaluation of inhibitors of the SARS-CoV-2 nsp3 macrodomain.Bioorg Med Chem. 2022 Aug 1;67:116788. doi: 10.1016/j.bmc.2022.116788. Epub 2022 May 11. Bioorg Med Chem. 2022. PMID: 35597097 Free PMC article.
-
Identifying SARS-CoV-2 antiviral compounds by screening for small molecule inhibitors of nsp13 helicase.Biochem J. 2021 Jul 16;478(13):2405-2423. doi: 10.1042/BCJ20210201. Biochem J. 2021. PMID: 34198322 Free PMC article.
-
Exploring the Conformational and Binding Dynamics of HMGA2·DNA Complexes Using Trapped Ion Mobility Spectrometry-Mass Spectrometry.J Am Soc Mass Spectrom. 2022 Jul 6;33(7):1103-1112. doi: 10.1021/jasms.2c00101. Epub 2022 Jun 10. J Am Soc Mass Spectrom. 2022. PMID: 35687119 Free PMC article.
-
Inhibition of HMGA2 binding to AT-rich DNA by its negatively charged C-terminus.Nucleic Acids Res. 2025 Jan 24;53(3):gkaf035. doi: 10.1093/nar/gkaf035. Nucleic Acids Res. 2025. PMID: 39873271 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

