Mass spectrometric quantitation of AGEs and enzymatic crosslinks in human cancellous bone
- PMID: 33139851
- PMCID: PMC7606603
- DOI: 10.1038/s41598-020-75923-8
Mass spectrometric quantitation of AGEs and enzymatic crosslinks in human cancellous bone
Abstract
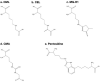
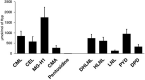
Advanced glycation end-products (AGEs) deteriorate bone strength. Among over 40 species identified in vivo, AGEs other than pentosidine were roughly estimated as total fluorescent AGEs (tfAGEs) due to technical difficulties. Using LC-QqTOF-MS, we established a system that enabled the quantitation of five AGEs (CML, CEL, MG-H1, CMA and pentosidine) as well as two mature and three immature enzymatic crosslinks. Human bone samples were collected from 149 patients who underwent total knee arthroplasty. Their clinical parameters were collected to investigate parameters that may be predictive of AGE accumulation. All the analytes were quantitated and showed significant linearity with high sensitivity and precision. The results showed that MG-H1 was the most abundant AGE, whereas pentosidine was 1/200-1/20-fold less abundant than the other four AGEs. The AGEs were significantly and strongly correlated with pentosidine, while showing moderate correlation with tfAGEs. Interestingly, multiple linear regression analysis revealed that gender contributed most to the accumulation of all the AGEs, followed by age, tartrate-resistant acid phosphatase-5b and HbA1c. Furthermore, the AGEs were negatively correlated with immature crosslinks. Mass spectrometric quantitation of AGEs and enzymatic crosslinks is crucial to a better understanding of ageing- and disease-related deterioration of bone strength.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone.Osteoporos Int. 2013 Sep;24(9):2441-7. doi: 10.1007/s00198-013-2319-4. Epub 2013 Mar 8. Osteoporos Int. 2013. PMID: 23471564 Free PMC article.
-
Non-invasive skin autofluorescence, blood and urine assays of the advanced glycation end product (AGE) pentosidine as an indirect indicator of AGE content in human bone.BMC Musculoskelet Disord. 2019 Dec 27;20(1):627. doi: 10.1186/s12891-019-3011-4. BMC Musculoskelet Disord. 2019. PMID: 31881872 Free PMC article.
-
Accumulation of carboxymethyl-lysine (CML) in human cortical bone.Bone. 2018 May;110:128-133. doi: 10.1016/j.bone.2018.01.028. Epub 2018 Feb 2. Bone. 2018. PMID: 29408699 Free PMC article.
-
New biomarkers of Maillard reaction damage to proteins.Nephrol Dial Transplant. 1996;11 Suppl 5:41-7. doi: 10.1093/ndt/11.supp5.41. Nephrol Dial Transplant. 1996. PMID: 9044306 Review.
-
[Determinants of bone quality and strength independent of bone remodeling].Clin Calcium. 2016 Jan;26(1):29-41. Clin Calcium. 2016. PMID: 26728528 Review. Japanese.
Cited by
-
Advanced Glycation End Products Associated With Cardiometabolic Biomarkers in Treated Human Immunodeficiency Virus Infection.Open Forum Infect Dis. 2021 Aug 16;8(10):ofab423. doi: 10.1093/ofid/ofab423. eCollection 2021 Oct. Open Forum Infect Dis. 2021. PMID: 34631914 Free PMC article.
-
Causative or associative: A critical review of the role of advanced glycation end-products in bone fragility.Bone. 2022 Oct;163:116485. doi: 10.1016/j.bone.2022.116485. Epub 2022 Jul 4. Bone. 2022. PMID: 35798196 Free PMC article. Review.
-
Fracture Toughness: Bridging the Gap Between Hip Fracture and Fracture Risk Assessment.Curr Osteoporos Rep. 2023 Jun;21(3):253-265. doi: 10.1007/s11914-023-00789-4. Epub 2023 Apr 27. Curr Osteoporos Rep. 2023. PMID: 37101058 Review.
-
The chemical language of protein glycation.Nat Chem Biol. 2025 Mar;21(3):324-336. doi: 10.1038/s41589-024-01644-y. Epub 2024 Jun 28. Nat Chem Biol. 2025. PMID: 38942948 Free PMC article. Review.
-
Mapping glycation and glycoxidation sites in collagen I of human cortical bone.BBA Adv. 2023 Jan 22;3:100079. doi: 10.1016/j.bbadva.2023.100079. eCollection 2023. BBA Adv. 2023. PMID: 37082268 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

